Lesson 4.2: Into the Chloroplast: How Photosyn- thesis Works
Lesson 4.2: Into the Chloroplast: How Photosyn- thesis Works
Lesson Objectives
Understand that hundreds of years of scientific exploration have contributed to our understanding of photosynthesis.
Explain the contributions of Van Helmont, Priestley, and Melvin Calvin to our under- standing of photosynthesis.
Describe the structure and function of chloroplasts, thylakoids, and pigments.
Explain how electron carrier molecules form electron transport chains.
Trace the flow of energy and materials through the Light Reactions, including chemios- mosis.
Trace the flow of energy and materials through The Calvin Cycle.
Compare and contrast C-3, C-4, and CAM pathways for carbon fixation.
Introduction
Life requires photosynthesis for fuel and for the oxygen to burn that fuel. Since the Industrial Revolution (late 18th and early 19th centuries), we humans have relied on products of ancient photosynthesis for enormous quantities of fossil fuel energy. And, knowingly or not, we have also benefited from photosynthesis to remove the carbon dioxide produced when we burn those fuels. So it may not surprise you that biologists have studied this critical process in great detail. The goals of this lesson are:
to discuss how scientists have explored this most important chemical reaction for life on earth
to encourage you to appreciate just a little of its intricate beauty, and
to understand how your own decisions and actions can influence the process of photo- synthesis.
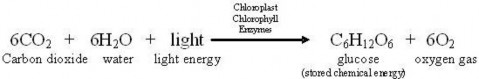
You’ve learned that a single chemical reaction represents the overall process of photosynthesis as demonstrated in the equation below.
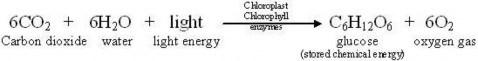
Although photosynthesis may seem straightforward in this form, such simplicity is deceiving for two reasons. First, the equation above summarizes dozens of individual chemical reactions
involving many intermediate compounds. And second, just discovering major players like CO2 and O2 was challenging, because our ordinary senses cannot detect these molecules in “thin air!”
How do we know that the chemical reaction in photosynthesis really happens? Two famous historical experiments help us begin to understand this process.

Figure 4.5: In the 17th century, Jan Van Helmont, a Flemish chemist, physiologist, and physician, weighed and potted a willow tree, showing that plants do not get food from the soil. (15)
In the 17th century, people who thought about it at all assumed that plants get their food from the soil. Many people, encouraged by sellers of “plant food,” still do. In 1638, Jan Baptist Van Helmont planted a 5 pound willow tree, like the one shown in Figure 4.5, in a 200 pound tub of soil. After 5 years of watering the plant, he weighed both again. The willow had gained over 160 pounds, but the soil had lost only 2 ounces. Van Helmont concluded that plants do not get their materials from soil, and inferred that they grow using materials from water (which he did not measure). As you know now, he was half right. Although soil provides important nutrients to plants, it supplies neither the energy nor the vast majority of the materials to build the plant. We must excuse him, because no one in the 17th century knew that carbon atoms form the basis of life, or that they float around in air in the form of carbon dioxide.
In the late 1770s, minister and natural philosopher Joseph Priestley burned a candle in a jar of air and observed that the candle burned out long before it ran out of wax. A similar experiment with a mouse resulted in the mouse’s death. Priestley suggested that animals, like candles, “injure” the air. Adding a mint plant, as shown in Figure 4.6, however, “restored” the air which had been “injured” by the mouse or the candle. Only later, after many chemistry experiments, did Priestley publish his discovery of “dephlogisticated air.” But in his studies of mice, plants, and candles, he had shown that plants produce, and animals consume, oxygen gas.
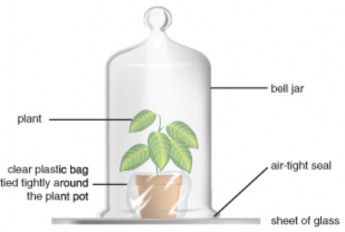
Figure 4.6: Joseph Priestly’s bell jar experiment. (8)
During the 20th century, we learned that photosynthesis involves much more than just the three reactants, the three necessary conditions, and the two products shown in the equation. Using powerful microscopes, we’ve narrowed the process to one type of organelle within the plant – the chloroplast. In the next section, you will learn in more detail just how plants, algae, and photosynthetic bacteria make food for us all “from thin air.” First, let’s look at the organelle in which the drama of photosynthesis takes place and meet some of the key actors.
For a detailed animation of the complete photosynthesis process, see http://vcell.ndsu. edu/animations/photosynthesis/first.htm.
Chloroplasts: Theaters for Photosynthesis
If you examine a single leaf of the aquatic plant Elodea, shown in Figure 4.7, under a micro- scope, you will see within each cell dozens of small green ovals. These are chloroplasts, the organelles which conduct photosynthesis in plants and algae. Chloroplasts closely resemble some types of bacteria and even contain their own circular DNA and ribosomes. In fact, the endosymbiotic theory holds that chloroplasts were once independently living bacteria (prokaryotes). So when we say that photosynthesis occurs within chloroplasts, we speak not only of the organelles within plants and algae, but also of some bacteria – in other words, virtually all photosynthetic autotrophs.

Figure 4.7: Elodea (above), like all plants and algae, consists of cells which contain organelles called chloroplasts (green ovals in the microphotograph below). If you look carefully at living cells through a microscope, you may see the chloroplasts moving slowly around the cell edges. The plant itself may not move, but this cyclosis hints at all the action within plant cells. (7)
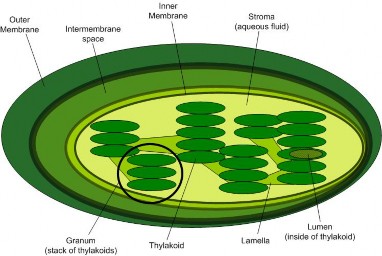
Both chloroplasts and photosynthetic bacteria contain neat stacks (grana) of flattened sac- shaped membrane compartments (thylakoids), made in turn of elaborate and highly or- ganized patterns of molecules which conduct photosynthesis, as shown in Figure 4.8. In addition to enzymes, two basic types of molecules - pigments and electron carriers – are key players.
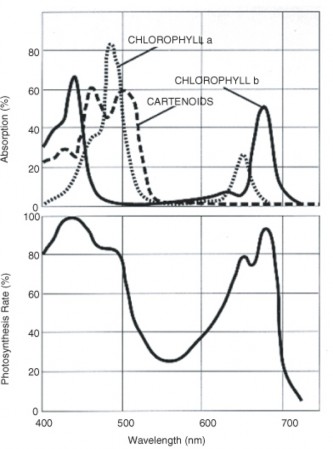
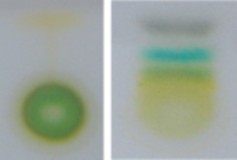
Pigment molecules, often arranged together with proteins in large, complex photosystems, absorb specific wavelengths of light energy and reflect others; therefore, they appear colored. The most common photosynthetic pigment is chlorophyll, which absorbs blue-violet and red wavelengths of light, and reflects green (Figure 4.9 and Figure 4.10). Accessory pigments absorb other colors of light and then transfer the energy to chlorophyll. These include xanthophylls (yellow) and carotenoids (orange).
Electron carrier molecules are usually arranged in electron transport chains (ETCs). These accept and pass along energy-carrying electrons in small steps (Figure 4.11). In this way, they produce ATP and NADPH, which temporarily store chemical energy. Electrons
in transport chains behave much like a ball bouncing down a set of stairs – a little energy is lost with each bounce. However, the energy “lost” at each step in an electron transport chain accomplishes a little bit of work, which eventually results in the synthesis of ATP.
Now that you’ve met some of the key players and explored the theater, let’s put them together to see how the process unfolds. We will divide the process into two basic sets of reactions, known as the light reactions and the Calvin cycle, which uses carbon dioxide. As you study the details, refer frequently to the chemical equation of photosynthesis. In the first stage, you’ll discover how chloroplasts transform light energy, and why we owe our ability to breathe to plants!
Photosynthesis Stage I: The Light Reactions: in which Chloro- plasts Capture Sunlight Chemical Energy…
Every second, the sun fuses over 600 million tons of hydrogen into 596 tons of helium, converting over 4 tons of helium (4.3 billion kg) into light and heat energy. Countless tiny packets of that light energy travel 93 million miles (150 million km) through space, and about 1% of the light which reaches the Earth’s surface participates in photosynthesis. Light is the source of energy for photosynthesis, and the first set of reactions which begin the process requires light – thus the name, Light Reactions, or Light-dependent Reactions.
When light strikes chlorophyll (or an accessory pigment) within the chloroplast, it energizes electrons within that molecule. These electrons jump up to higher energy levels; they have absorbed or captured, and now carry, that energy. High-energy electrons are “excited.” Who wouldn’t be excited to hold the energy for life?
…And Change the Rules of Chemistry for Life!
The excited electrons leave chlorophyll to participate in further reactions, leaving the chloro- phyll “at a loss”; eventually they must be replaced. That replacement process also requires light, working with an enzyme complex to split water molecules. In this process of photoly- sis (“splitting by light”), H2O molecules are broken into hydrogen ions, electrons, and oxygen atoms. The electrons replace those originally lost from chlorophyll. Hydrogen ions and the high-energy electrons from chlorophyll will carry on the energy transformation drama after the Light Reactions are over.
The oxygen atoms, however, form oxygen gas, which is a waste product of photosynthesis (Figure 4.12). The oxygen given off supplies most of the oxygen in our atmosphere. Be- fore photosynthesis evolved, Earth’s atmosphere lacked oxygen altogether, and this highly reactive gas was toxic to the many organisms living at the time. Something had to change! Most contemporary organisms rely on oxygen for efficient respiration. So plants don’t just “restore” the air, as Priestley suggested. They also had a major role in creating it!
To summarize, chloroplasts “capture” sunlight energy in two ways. Light “excites” electrons in pigment molecules, and light provides the energy to split water molecules, providing more electrons as well as hydrogen ions.
Now let’s follow those excited electrons…

How Do Chloroplasts Convert Light Energy to Chemical Energy?
Excited electrons which have absorbed light energy are unstable. However, the highly or- ganized electron carrier molecules embedded in chloroplast membranes order the flow of these electrons, directing them through electron transport chains (ETCs). At each transfer, small amounts of energy released by the electrons are captured and put to work or stored. Some is also lost as heat with each transfer, but overall the light reactions are extremely efficient at capturing light energy and transforming it to chemical energy.
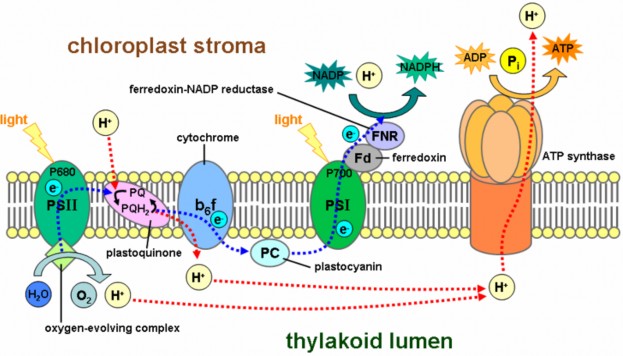
Two sequential transport chains harvest the energy of excited electrons, as shown in Figure
4.13.
First, they pass down an ETC which captures their energy and uses it to pump hydrogen ions by active transport into the thylakoids. These concentrated ions store potential energy by forming achemiosmotic or electrochemical gradient – a higher concen- tration of both positive charge and hydrogen inside the thylakoid than outside. (The gradient formed by the H+ ions is known as a chemiosmotic gradient.) Picture this energy buildup of H+ as a dam holding back a waterfall. Like water flowing through a hole in the dam, hydrogen ions “slide down” their concentration gradient through a membrane protein which acts as both ion channel and enzyme. As they flow, the ion channel/enzyme ATP synthase uses their energy to chemically bond a phosphate group to ADP, making ATP.
Light re-energizes the electrons, and they travel down a second electron transport chain (ETC), eventually bonding hydrogen ions to NADP+ to form a more stable energy storage molecule, NADPH. NADPH is sometimes called “hot hydrogen,” and its en- ergy and hydrogen atoms will be used to help build sugar in the second stage of photosynthesis.
−→ −→ −→
−→ −→
Figure 4.13: Membrane architecture: The large colored carrier molecules form electron trans- port chains which capture small amounts of energy from excited electrons in order to store it in ATP and NADPH. Follow the energy pathways: light electrons NADPH (blue line) and light electrons concentrated H+ ATP (red line). Note the intricate organization of the chloroplast. (10)
NADPH and ATP molecules now store the energy from excited electrons – energy which was originally sunlight – in chemical bonds. Thus chloroplasts, with their orderly arrangement of pigments, enzymes, and electron transport chains, transform light energy into chemical energy. The first stage of photosynthesis – light-dependent reactions or simply “light reactions” – is complete.
Photosynthesis Stage II: The Calvin Cycle - Making Food “From Thin Air”
You’ve learned that the first, light-dependent stage of photosynthesis uses two of the three reactants - water and light - and produces one of the products - oxygen gas (a waste product of this process). All three necessary conditions are required – chlorophyll pigments, the chloroplast “theater,” and enzyme catalysts. The first stage transforms light energy into chemical energy, stored to this point in molecules of ATP and NADPH. Look again at the overall equation below. What is left?

Waiting in the wings is one more reactant – carbon dioxide, and yet to come is the star product which is food for all life – glucose. These key players perform in the second act of the photosynthesis drama, in which food is “made from thin air!”
The second stage of photosynthesis can proceed without light, so its steps are sometimes called “light-independent” or “dark” reactions. Many biologists honor the scientist, Melvin Calvin, who won a 1961 Nobel Prize for working out this complex set of chemical reactions, naming it the Calvin Cycle.
The Calvin Cycle has two parts. First carbon dioxide is ”fixed.” Then ATP and NADPH from the Light Reactions provide energy to combine the fixed carbons to make sugar.
Carbon Dioxide is “Fixed”
Why does carbon dioxide need to be fixed? Was it ever broken?
Life on Earth is carbon-based. Organisms need not only energy but also carbon atoms for building bodies. For nearly all life, the ultimate source of carbon is carbon dioxide (CO2), an inorganic molecule. CO2, as you saw in Figure 4.14, makes up .038% of the Earth’s atmosphere.
Animals and most other heterotrophs cannot take in CO2 directly. They must eat other organisms or absorb organic molecules to get carbon. Only autotrophs can build low- energy inorganic CO2 into high-energy organic molecules like glucose. This process is carbon fixation.
Plants have evolved three pathways for carbon fixation.
The most common pathway combines one molecule of CO2 with a 5-carbon sugar called ribu- lose biphosphate (RuBP). The enzyme which catalyzes this reaction (nicknamed RuBisCo) is the most abundant enzyme on earth! The resulting 6-carbon molecule is unstable, so it immediately splits into two 3-carbon molecules. The 3 carbons in the first stable molecule of this pathway give this largest group of plants the name “C-3.”
Dry air, hot temperatures, and bright sunlight slow the C-3 pathway for carbon fixation. This is because stomata, tiny openings under the leaf which normally allow CO2 to enter and O2 to leave, must close to prevent loss of water vapor (Figure 4.14). Closed stomata lead to a shortage of CO2. Two alternative pathways for carbon fixation demonstrate biochemical adaptations to differing environments.
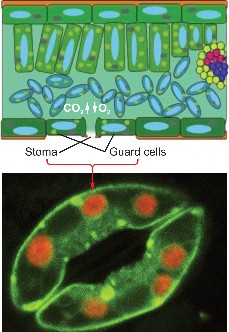
Plants such as corn solve the problem by using a separate compartment to fix CO2. Here CO2 combines with a 3-carbon molecule, resulting in a 4-carbon molecule. Because the first stable organic molecule has four carbons, this adaptation has the name C-4. Shuttled away from the initial fixation site, the 4-carbon molecule is actually broken back down into CO2, and when enough accumulates, Rubisco fixes it a second time! Compartmentalization allows efficient use of low concentrations of carbon dioxide in these specialized plants.
Cacti and succulents such as the jade plant avoid water loss by fixing CO2 only at night. These plants close their stomata during the day and open them only in the cooler and more humid nighttime hours. Leaf structure differs slightly from that of C-4 plants, but the fixation pathways are similar. The family of plants in which this pathway was discovered gives the pathway its name, Crassulacean Acid Metabolism, or CAM (Figure 4.15). All three carbon fixation pathways lead to the Calvin Cycle to build sugar.

How Does the Calvin Cycle Store Energy in Sugar?
As Melvin Calvin discovered, carbon fixation is the first step of a cycle. Like an electron transport chain, the Calvin cycle, shown in Figure 4.16, transfers energy in small, controlled steps. Each step pushes molecules uphill in terms of energy content. Recall that in the electron transfer chain, excited electrons lose energy to NADPH and ATP. In the Calvin Cycle, NADPH and ATP formed in the light reactions lose their stored chemical energy to build glucose.
Use the diagram below to identify the major aspects of the process:
the general cycle pattern
the major reactants
the products
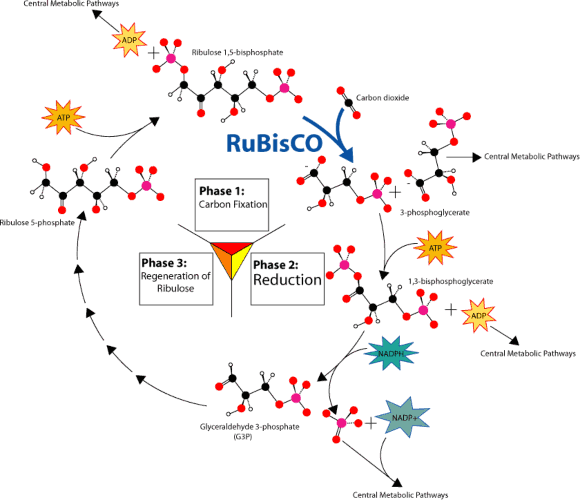
Figure 4.16: Overview of the Calvin Cycle Pathway. (13)
First, notice where carbon is fixed by the enzyme nicknamed Rubisco. In C-3, C-4, and CAM plants, CO2 enters the cycle by joining with 5-carbon ribulose bisphosphate to form a 6-carbon intermediate, which splits (so quickly that it isn’t even shown!) into two 3-carbon molecules.
Now look for the points at which ATP and NADPH (made in the light reactions) add chemical energy (“Reduction” in the diagram) to the 3-carbon molecules. The resulting “half-sugars” can enter several different metabolic pathways. One recreates the original 5- carbon precursor, completing the cycle. A second combines two of the 3-carbon molecules to form glucose, universal fuel for life.
The cycle begins and ends with the same molecule, but the process combines carbon and energy to build carbohydrates – food for life.
So – how does photosynthesis store energy in sugar? Six “turns” of the Calvin cycle use chemical energy from ATP to combine six carbon atoms from six CO2 molecules with 12 “hot hydrogens” from NADPH. The result is one molecule of glucose, C6H12O6.
Lesson Summary
The single chemical equation below represents the overall process of photosynthesis as well as summarizes many individual chemical reactions that were understood only after hundreds of years of scientific exploration.
Chloroplasts are the organelles where the process of photosynthesis takes place in plants and algae.
Chloroplasts resemble blue green bacteria, containing their own DNA and ribosomes.
The Endosymbiotic Theory holds that chloroplasts once were independent prokary- otic cells, but were engulfed by other larger prokaryotes, forming the first eukaryotic cells.
Chloroplasts are made of membranes, which enclose stacks of membrane sacs called thylakoids.
The membranes sequence pigments and electron carrier molecules for efficient photosynthesis.
Thylakoids create compartments, which allow concentration gradients to store energy.
Pigment molecules absorb specific wavelengths (colors) of light; chlorophyll is the primary pigment in photosynthesis.
Electron carrier molecules form electron transport chains, which transfer energy in small steps so that the energy can be stored or used for work.
Photosynthesis consists of two groups of chemical reactions: the Light Reactions and the Calvin Cycle.
Light Reactions transform energy from sunlight into chemical energy, and produce and release oxygen gas.
When light strikes pigment molecules, electrons absorb its energy and are excited.
Light also provides energy to split water molecules into electrons, hydrogen ions, and oxygen gas.
The oxygen gas is released as “waste”, but it is the source of the oxygen in Earth’s atmosphere.
Two pathways capture the energy from excited electrons as chemical energy stored in the bonds of molecules; both pathways involve electron transport chains.
One produces NADPH molecules, which stores energy and “hot hydrogen”.
A second pumps hydrogen ions into the thylakoids, forming an electrochemical gradient whose energy builds ATP molecules. This is “chemiosmosis”.
The Calvin Cycle uses the NADPH and ATP from the Light Reactions to “fix” carbon and produce glucose.
Stomata underneath plant leaves allow gases (CO2, H2O, and O2) to enter and exit the leaf interior.
Carbon dioxide enters the Calvin Cycle when an enzyme nicknamed “Rubisco” attaches it to a 5-carbon sugar. The unstable 6-carbon compound immediately breaks into two 3-carbon compounds, which continue the cycle.
Most plants fix CO2 directly with this pathway, so they are called C-3 plants.
Some plants have evolved preliminary fixation pathways, which help them conserve wa- ter in hot, dry habitats, but eventually the carbon enters the cycle along the “Rubisco” pathway.
C-4 plants such as corn use a 3-carbon carrier to compartmentalize initial carbon fixation in order to concentrate CO2 before sending it on to Rubisco.
CAM plants such as jade plants and some cacti open their stomata for preliminary CO2 fixation only at night.
In the Calvin Cycle, the fixed CO2 moves through a series of chemical reactions, gaining a small amount of energy (or “hot hydrogens”) from ATP or NADPH at each step.
Six turns of the cycle process 6 molecules of carbon dioxide and 12 “hot hydrogens” to produce a single molecule of glucose.
The cycle begins and ends with the same 5-carbon molecule, but the process stores chemical energy in food for nearly all life.
Summary Animations
These interactive web sites depicts each step of photosynthesis in great detail.
http://www.johnkyrk.com/photosynthesis.html http://www.johnkyrk.com/photosynthesisdark.html
Review Questions
Explain how the following actions would affect photosynthesis:
Further Reading / Supplemental Links
Summarize Jan Van Helmont’s willow tree experiment. State his conclusion and the in- ference he made after his experiment, and explain how his data supports each. Finally, relate his findings to what we know today about the overall process of photosynthesis.
Using the overall equation for photosynthesis, explain which components relate to J.B. Priestley’s observation that “Plants restore the air that animals injure.”
Explain how the structure of a chloroplast – its membranes and thylakoids – makes its function – the chemical reactions of photosynthesis – more efficient.
Summarize the Endosymbiotic Theory. What evidence related to chloroplasts supports this theory?
Name the two stages (sets of reactions) which make up the process of photosynthesis.
Match the major events with the stage of photosynthesis in which they occur. StagesLight Reactions
Calvin Cycle
Carbon dioxide is fixed.
Electrons in chlorophyll jump to higher energy levels.
Glucose is produced.
NADPH and ATP are produced.
NADPH and ATP are used.
Oxygen gas is released.
Water is split.
Use your understanding of pigments to explain why the living world appears green. Then think a little further and offer a hypothesis to explain why the world is not black!
Explain the value of cycles of chemical reactions, such as the Calvin Cycle.
Explain how their various methods of carbon fixation adapt C-3, C-4, and CAM plants to different habitats.
We humans depend on photosynthesis, and our actions in turn affect photosynthesis. Explain how humans depend on photosynthesis for:
food
building materials for furniture and homes
fuel for vehicles, heat, and electricity
breathable air
We may clear-cut a forest for timber and parking lot space
When we burn fossil fuels for transportation or heat, we release CO2 into the atmosphere
When we dam up and overuse water in a certain area, the area water table drops
Graham Kent, “Light Reactions in Photosynthesis” Animation. Bio 231 Cell Biology Lab, October 2004. Available on the web at:
http://www.science.smith.edu/departments/Biology/Bio231/ltrxn.html.
Illustrator: Thomas Porostocky; Writer: Lee Billings; Map data adapted from MODIS observations by NASA’s Terra and Aqua satellites; Graph data and reference: Biology, 4th ed., Neil A. Campbell, Benjamin/Cummings Publishing Company, 1996. “Crib Sheet #10, Photosynthesis.” Seed Magazine, August 2007. Available on the web at:
http://www.seedmagazine.com/news/uploads/cribsheet10.gif.
John Mynett, “Photosynthesis Animations.” Biology4All, 01 January 2002. Available on the web at:
http://www.biology4all.com/resources_library/details.asp?ResourceID=43
Kenneth R. Spring, Thomas J. Fellers, and Michael W. Davidson, “Introduction to Light and Energy.” Molecular Expressions Optical Microscopy Primer. The Physics of Light and Energy, Last modified Aug 23, 2005. Available on the web at:
http://micro.magnet.fsu.edu/primer/lightandcolor/lightandenergyintro.html.
Laurence A. Moran and Pearson Prentice Hall, “Fixing Carbon: The Rubisco reaction,” 10 July 2007. Sandwalk: Strolling with a Skeptical Biochemist,
http://sandwalk.blogspot.com/2007/07/fixing-carbon-rubisco-reaction.html.
“Photosynthesis”, “Electron Transport Chain” and “ATP Synthase” Animations. Vir- tual Cell Animation Collection, Molecular and Cellular Biology Learning Center, no date given. Available on the web at:
http://vcell.ndsu.nodak.edu/animations/photosynthesis/index.htm.
Vocabulary
accessory pigment A molecule which absorbs colors of light other than blue-violet and red, and then transfers the energy to chlorophyll.
ATP synthase Ion channel and enzyme complex that chemically bonds a phosphate group to ADP, making ATP as H+ ions flow through the ion channel.
Calvin Cycle The second stage of photosynthesis, which can proceed without light, so its steps are sometimes called “light-independent” or “dark” reactions; results in the formation of a sugar.
carbon fixation The process which converts carbon dioxide in the air to organic molecules, as in photosynthesis.
chlorophyll The primary pigment of photosynthesis.
chloroplast The organelle in plant and algal cells where photosynthesis takes place.
electron carrier A molecule which transfers energy-carrying electrons within an electron transport chain.
electron transport chain (ETC) A series of electron-carrying molecules which accept and pass along energy-carrying electrons in small steps, allowing the energy lost at each transfer to be captured for storage or work.
endosymbiotic theory The theory which states that chloroplasts and mitochondria orig- inated as independent prokaryotic cells which were engulfed by larger prokaryotic cells to form the first eukaryotic cells.
glucose The carbohydrate product of photosynthesis; serves as the universal fuel for life.
light-dependent reactions The first set of reactions of photosynthesis; requires sunlight; also called the light reactions.
NADPH An energy carrier molecule produced in the light reactions of photosynthesis; used to build sugar in the Calvin cycle.
photolysis The light reaction process of splitting water molecules into electrons, hydrogen ions, and oxygen gas.
photosynthesis The process by which plants, algae, and some bacteria transform sunlight into chemical energy and use it to produce carbohydrate food and oxygen for almost all life.
photosystem A cluster of proteins and pigments found in chloroplasts and active in pho- tosynthesis.
pigment A molecule which absorbs specific wavelengths of light energy and reflects others and therefore appears colored.
RuBisCo The enzyme that combines one molecule of CO2 with a 5-carbon sugar called ribulose biphosphate (RuBP); the most abundant enzyme on earth.
stomata (singular stoma) Openings on the underside of a leaf which allow gas exchange and transpiration.
thylakoid Flattened sac-shaped compartment within a chloroplast, made of membranes embedded with molecules which carry out photosynthesis.
Points to Consider
Recall Priestley’s early observation that plants “restore the air.” Name some ways that plants and algae affect the atmosphere.
Which of your own activities affect photosynthesis? Think “globally” in addition to “locally” and add large-scale human activities to your list. Are there any changes you could make in your life which could promote photosynthesis and a healthy atmosphere?
You learned in this chapter that plants make ”food” which life needs for energy. But is it usable energy? Or does it need to be converted into some other type of energy? What do you think and why?
Image Sources
http://commons.wikimedia.org/wiki/File: Chromatography_of_chlorophyll_-_Step_6.jpg. CC-BY-SA 2.5.
Alex Costa,Gross L, PLoS Biology Vol. 4/10/2006, ed. 358.
http://dx.doi.org/10.1371/journal.pbio.0040358. Public Domain,CC-BY.
Antje Boetius. http://biology.plosjournals.org/perlserv/?request= get-document&doi=10.1371/journal.pbio.0030102. CC-BY.
http://en.wikipedia.org/wiki/Image:Chlorophyll_structure.png. Public Domain.
http://www.flickr.com/photos/dropandroll/368377017. Creative Commons.
CK-12 Foundation. Joseph Priestly’s bell jar experiment.. CC-BY-SA.
http://www.flickr.com/photos/lobo235/76154752/ http://www.flickr.com/photos/jylcat/562393266/. CC-BY 2.0.
http://en.wikipedia.org/wiki/Image:Thylakoid_membrane.png. Public Domain.
http://commons.wikimedia.org/wiki/Image:Chloroplast-new.jpg. Public Domain.
http://en.wikipedia.org/wiki/Image:The_Earth_seen_from_Apollo_17.jpg. Public Domain.
Mike Jones. Overview of the Calvin Cycle Pathway.. CC-BY-SA 2.5.
http://commons.wikimedia.org/wiki/File:Kelp_300.jpg http:
//upload.wikimedia.org/wikipedia/commons/6/64/Anabaena_sperica.jpeg. (a)Public Domain (b)Public Domain (c)Creative Commons.
CK-12 Foundation. http://en.wikipedia.org/wiki/Image:Par_action_spectrum.gif. CC-BY-SA 2.0.
- Log in or register to post comments
- Email this page
