
CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web- based collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and distribution of high quality educational content that will serve both as core text as well as provide an adaptive environment for learning.
Copyright ©2009 CK-12 Foundation
This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License. To view a copy of this license, visit http://creativecommons.org/licenses/ by-sa/3.0/us/ or send a letter to Creative Commons, 171 Second Street, Suite 300, San Francisco, California, 94105, USA.

Foundations of Life Science 1
1.1 Lesson 1.1: Nature of Science . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Lesson 1.2: Communicating Ideas 25
Lesson 1.3: Tools and Techniques 53
Lesson 1.4: Principles of Biology 71
Lesson 2.1: Matter 93
Lesson 2.2: Organic Compounds 103
Lesson 2.3: Chemical Reactions 119
Lesson 2.4: Water 129
Cell Structure and Function 145
Lesson 3.1: Introduction to Cells 145
Lesson 3.2: Cell Structures 161
Lesson 3.3: Cell Transport and Homeostasis 187
Photosynthesis 209
Lesson 4.1: Energy for Life: An Overview of Photosynthesis 209
Lesson 4.2: Into the Chloroplast: How Photosynthesis Works 224
Lesson 5.1: Powering the Cell: Cellular Respiration and Glycolysis 245
Lesson 5.2: Into the Mitochondrion: Making ATP with Oxygen 262
Lesson 5.3: Anaerobic Respiration: ATP, New Fuels, and Yogurt without Oxygen 274
Cell Division and Reproduction 289
Lesson 6.1: Chromosomes and the Cell Cycle 289
Lesson 6.2: Meiosis 303
Lesson 7.1: Mendel’s Investigations 317
Lesson 7.2: Mendelian Inheritance 331
Lesson 8.1: DNA and RNA 347
Lesson 8.2: Protein Synthesis 365
Lesson 8.3: Mutation 381
Lesson 8.4: Regulation of Gene Expression 394
Human Genetics 407
Lesson 9.1: Human Chromosomes and Genes 407
Lesson 9.2: Human Inheritance 418
Biotechnology 447
Lesson 10.1: DNA Technology 447
Lesson 10.2: Biotechnology 459
History of Life 481
Lesson 11.1: Studying the History of Life 481
Lesson 11.2: Early Life 503
Lesson 11.3: Multicellular Life 516
www.ck12.org ii
Lesson 12.1: Darwin and The Theory of Evolution 551
Lesson 12.2: Evidence for Evolution 570
Lesson 12.3: Evolution Continues Today - Can We Control It? 590
Lesson 13.1: Genetics of Populations 609
Lesson 13.2: Genetic Change in Populations 624
Lesson 13.3: The Origin of Species 652
Classification 675
Lesson 14.1: Form and Function 675
Lesson 14.2: Phylogenetic Classification 686
Lesson 14.3: Modern Classification Systems 696
Lesson 15.1: The Science of Ecology 709
Lesson 15.2: Flow of Energy 720
Lesson 15.3: Recycling Matter 735
Biomes, Ecosystems, and Communities 749
Lesson 16.1: Biomes 749
Lesson 16.2: Terrestrial Biomes 758
Lesson 16.3: Aquatic Biomes 768
Lesson 16.4: Community Interactions 785
Populations 801
Lesson 17.1: Characteristics of Populations 801
Lesson 17.2: Population Dynamics 813
Lesson 17.3: Human Population Growth: Doomsday, Cornucopia, or Some- where in Between? 839
Lesson 18.1: The Biodiversity Crisis 863
Lesson 18.2: Natural Resources 904
Lesson 18.3: Natural Resources II: The Atmosphere 925
Lesson 18.4: Climate Change 948
The Human Body 971
Lesson 19.1: Organization of the Human Body 971
Lesson 19.2: Homeostasis and Regulation 982
Nervous and Endocrine Systems 995
Lesson 20.1: The Nervous System 995
Lesson 20.2: The Endocrine System 1050
Skeletal, Muscular, and Integumentary Systems 1091
Lesson 21.1: Skeletal System 1091
Lesson 21.2: Muscular System 1119
Lesson 21.3: Integumentary System 1147
Circulatory and Respiratory Systems 1169
Lesson 22.1: Circulatory System 1169
Lesson 22.2: Blood 1202
Lesson 22.3: Respiratory System 1218
Digestive and Excretory Systems 1235
Lesson 23.1: Food and Nutrients 1235
Lesson 23.2: Digestive System 1255
Lesson 23.3: Excretory System 1270
Immune System and Disease 1285
Lesson 24.1: Nonspecific Defenses 1285
Lesson 24.2: Immune Response 1292
www.ck12.org iv
Lesson 24.3: Immune System Diseases 1307
Lesson 24.4: Environmental Problems and Human Health 1317
Reproductive System and Human Development 1331
Lesson 25.1: Male Reproductive System 1331
Lesson 25.2: Female Reproductive System 1340
Lesson 25.3: Fertilization, Gestation, and Development 1355
Lesson 25.4: Sexually Transmitted Diseases 1373
Appendix: Biology I 1383
Investigation and Experimentation Activities 1383
www.ck12.org vi
List the principles that should guide scientific research.
Examine a scientist’s view of the world.
Outline a set of steps that might be used in the scientific method of investigating a problem.
Explain why a control group is used in an experiment.
Outline the role that reasoning plays in examining hypotheses.
Examine the function of the independent variable in an experiment.
Define what is meant by a theory and compare this to the meaning of hypothesis.
The goal of science is to learn how nature works by observing the physical world, and to understand it through research and experimentation. Science is a distinctive way of learning about the natural world through observation, inquiry, formulating and testing hypotheses, gathering and analyzing data, and reporting and evaluating findings. We are all part of an amazing and mysterious phenomenon called ”Life” that thousands of scientists everyday are trying to better explain. And it’s surprisingly easy to become part of this great discovery! All you need is your natural curiosity and an understanding of how people use the process of science to learn about the world.
Science involves objective, logical, and repeatable attempts to understand the principles and forces working in the natural universe. Science is from the Latin word, scientia, which means “knowledge.” Good science is an ongoing process of testing and evaluation. One of the intended benefits for students taking a biology course is that they will become more familiar with the scientific process.
Humans are naturally interested in the world we live in. Young children constantly ask ”why” questions. Science is a way to get some of those “whys” answered. When we shop for groceries, we are carrying out a kind of scientific experiment (Figure 11.1). If you like Brand X of salad dressing, and Brand Y is on sale, perhaps you try Brand Y. If you like Y you may buy it again even when it is not on sale. If you did not like Brand Y, then no sale will get you to try it again. To find out why a person makes a particular purchasing choice, you might examine the cost, ingredient list, or packaging of the two salad dressings.

There are many different areas of science, or scientific disciplines, but all scientific study involves:
asking questions
making observations
being skeptical about ideas or results
Skepticism is an attitude of doubt about the truthfulness of claims that lack empirical evi- dence. Scientific skepticism, also referred to as skeptical inquiry, questions claims based on their scientific verifiability rather than accepting claims based on faith or anecdotes. Sci- entific skepticism uses critical thinking to analyze such claims and opposes claims which lack scientific evidence.
Science is based on the analysis of things that humans can observe either by themselves through their senses, or by using special equipment. Science therefore cannot explain any- thing about the natural world that is beyond what is observable by current means. The term supernatural refers to entities, events, or powers regarded as being beyond nature, in that such things cannot be explained by scientific means. They are not measurable or observable in the same way the natural world is, and so considered to be outside the realm of scientific examination.
When a natural occurrence which was once considered supernatural is understood in the terms of natural causes and consequences, it has a scientific explanation. For example, the flickering lights sometimes seen hovering over damp ground on still evenings or nights are commonly called Will-o’-the-wisp. This phenomena looks like a lamp or flame, and is sometimes said to move away if approached. A great deal of folklore surrounds the legend, such as the belief that the lights are lost souls or fairies attempting to lead travelers astray. However, science has offered several potential explanations for Will-o’-the-wisp from burning marsh gases to glowing fungi or animals that glow in a similar way to lightning bugs.
There is no fixed set of steps that scientists always follow and there is no single path that leads to scientific knowledge. There are, however, certain features of science that give it a very specific way of investigating something. You do not have to be a professional scientist to think like a scientist. Everyone, including you, can use certain features of scientific thinking to think critically about issues and situations in everyday life.
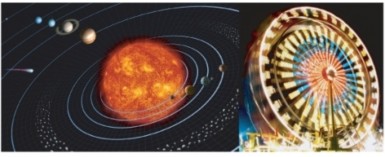
Science assumes that the universe is a vast single system in which the basic rules are the same, and thus nature, and what happens in nature, can be understood. Things that are learned from studying one part of the universe can be applied to other parts of the universe. For example, the same principles of motion and gravitation that explain the motion of falling objects on Earth also explain the orbit of the planets around the sun, and galaxies, as shown in Figure 1.2. As discussed below, as more and more information and knowledge is collected and understood, scientific ideas can change, still scientific knowledge usually stands the test of time. Science, however, cannot answer all questions.
Science presumes that events in the universe happen in patterns that can be understood by careful study. Scientists believe that through the use of the mind, and with the help of instruments that extend the human senses, people can discover patterns in all of nature that can help us understand the world and the universe.
Science is a process for developing knowledge. Change in knowledge about the natural world is expected because new observations may challenge the existing understanding of nature. No matter how well one theory explains a set of observations, it is possible that another theory may fit just as well or better, or may fit a still wider range of observations. In science, the testing and improving of theories goes on all the time. Scientists know that even if there is no way to gain complete knowledge about something, an increasingly accurate understanding of nature will develop over time.
The ability of scientists to make more accurate predictions about the natural world, from determining how a cancerous tumor develops a blood supply, to calculating the orbit of an asteroid, provides evidence that scientists are gaining an understanding of how the world works.
Continuity and stability are as much characteristics of science as change is. Although scien- tists accept some uncertainty as part of nature, most scientific knowledge stands the test of time. A changing of ideas, rather than a complete rejection of the ideas, is the usual practice in science. Powerful ideas about nature tend to survive, grow more accurate and become
www.ck12.org 4
more widely accepted.
For example, in developing the theory of relativity, Albert Einstein did not throw out Issac Newton’s laws of motion but rather, he showed them to be only a small part of the bigger, cosmic picture. That is, the Newtonian laws of motion have limited use within our more general concept of the universe. For example, the National Aeronautics and Space Adminis- tration (NASA) uses the Newtonian laws of motion to calculate the flight paths of satellites and space vehicles.
There are many things that cannot be examined in a scientific way. There are, for instance, beliefs that cannot be proved or disproved, such as the existence of supernatural powers, supernatural beings, or the meaning of life. In other cases, a scientific approach to a question and a scientific answer may be rejected by people who hold to certain beliefs.
Scientists do not have the means to settle moral questions surrounding good and evil, or love and hate, although they can sometimes contribute to the discussion of such issues by identifying the likely reasons for certain actions by humans and the possible consequences of these actions.
It can be difficult sometimes to define research methods in a way that will clearly distinguish science from non-science. However, there is a set of core principles that make up the “bones” of scientific research. These principles are widely accepted within the scientific community and in academia.
We learned earlier in this lesson that there is no fixed set of steps that scientists always follow during an investigation. Similarly, there is no single path that leads scientists to knowledge. There are, however, certain features of science that give it a very specific way of investigating things.
Scientific investigations examine, gain new knowledge, or build on previous knowledge about phenomena. A phenomenon, is any occurrence that is observable, such as the burning match shown in Figure 1.3. A phenomenon may be a feature of matter, energy, or time. For example, Isaac Newton made observations of the phenomenon of the moon’s orbit. Galileo Galilei made observations of phenomena related to swinging pendulums. Although procedures vary from one field of scientific inquiry to another, certain features distinguish scientific inquiry from other types of knowledge. Scientific methods are based on gathering observable, empirical (produced by experiment or observation), and measurable evidence that is critically evaluated.
A hypothesis is a suggested explanation based on evidence that can be tested by observation

Figure 1.3: The combustion of this match is an observable event and therefore a phenomenon. (49)
www.ck12.org 6
or experimentation. Experimenters may test and reject several hypotheses before solving a problem. A hypothesis must be testable; it gains credibility by being tested over and over again, and by surviving several attempts to prove it wrong.
The scientific method is not a step by step, linear process. It is a way of learning about the world through the application of knowledge. Scientists must be able to have an idea of what the answer to an investigation is. Scientists will often make an observation and then form a hypothesis to explain why a phenomenon occurred. They use all of their knowledge and a bit of imagination in their journey of discovery.
Scientific investigations involve the collection of data through observation, the formation and testing of hypotheses by experimentation, and analysis of the results that involves reasoning.
Scientific investigations begin with observations that lead to questions. We will use an everyday example to show what makes up a scientific investigation. Imagine that you walk into a room, and the room is dark.
You observe that the room appears dark, and you question why the room is dark.
In an attempt to find explanations to this phenomenon, you develop several different hypotheses. One hypothesis might state that the room does not have a light source at all. Another hypothesis might be that the lights are turned off. Still, another might be that the light bulb has burnt out. Worse yet, you could be going blind.
To discover the answer, you experiment. You feel your way around the room and find a light switch and turn it on. No light. You repeat the experiment, flicking the switch back and forth; still nothing.
This means your first two hypotheses, that the room is dark because (1) it does not have a light source; and (2) the lights are off, have been rejected.
You think of more experiments to test your hypotheses, such as switching on a flashlight to prove that you are not blind.
In order to accept your last remaining hypothesis as the answer, you could predict that changing the light bulb will fix the problem. If your predictions about this hypothesis succeed (changing the light bulb fixes the problem), the original hypothesis is valid and is accepted.
However, in some cases, your predictions will not succeed (changing the light bulb does not fix the problem), and you will have to start over again with a new hypothesis. Perhaps there is a short circuit somewhere in the house, or the power might be out.
The general process of a scientific investigation is summed up in Figure 1.4.
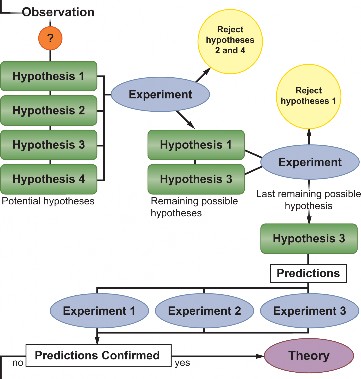
www.ck12.org 8
Table 1.1: Common Terms Used in Scientific Investigations
![]()
Term Definition
![]()
Scientific Method The process of scientific investigation.
Observation The act of noting or detecting phenomenon by the senses. For example, taking measure- ments is a form of observation.
Hypotheses A suggested explanation based on evidence that can be tested by observation or exper- imentation.
Scientific Reasoning The process of looking for scientific reasons for observations.
Experiment A test that is used to rule out a hypothesis or validate something already known.
Rejected Hypothesis An explanation that is ruled out by experi- mentation.
Confirmed Hypothesis An explanation that is not ruled out by re- peated experimentation, and makes predic- tions that are shown to be true.
Inference Developing new knowledge based upon old knowledge.
Theory A widely accepted hypothesis that stands the test of time. Theories are often tested, and usually not rejected.
![]()
Scientists first make observations that raise questions. An observation is the act of noting or detecting phenomenon through the senses. For example, noting that a room is dark is an observation made through sight.
In order to explain the observed phenomenon, scientists develop a number of possible ex- planations, or hypotheses. A hypothesis is a suggested explanation for a phenomenon or a suggested explanation for a relationship between many phenomena. Hypotheses are al- ways based on evidence that can be tested by observation or experimentation. Scientific investigations are required to test hypotheses. Scientists mostly base hypotheses on prior observations or on extensions of existing scientific explanations.
A hypothesis is not really an educated guess. To define a hypothesis as ”an educated guess”
is like calling a tricycle a ”vehicle with three.” The definition leaves out the concept’s most important and characteristic feature: the purpose of hypotheses. People generate hypothe- ses as early attempts to explain patterns observed in nature or to predict the outcomes of experiments. For example, in science, one could correctly call the following statement a hy- pothesis: identical twins can have different personalities because the environment influences personality.
Scientific methods require hypotheses that are falsifiable, that is, they must be framed in a way that allows other scientists to prove them false. Proving a hypothesis to be false is usually done by observation. However, confirming or failing to falsify a hypothesis does not necessarily mean the hypothesis is true.
For example, a person comes to a new country and observes only white sheep. This person might form the hypothesis: “All sheep in this country are white.” This statement can be called a hypothesis, because it is falsifiable - it can be tested and proved wrong; anyone could falsify the hypothesis by observing a single black sheep, shown in Figure 1.5. If the experimental uncertainties remain small (could the person reliably distinguish the observed black sheep from a goat or a small horse), and if the experimenter has correctly interpreted the hypothesis, finding a black sheep falsifies the ”only white sheep” hypothesis. However, you cannot call a failure to find non-white sheep as proof that no non-white sheep exist.

www.ck12.org 10
Any useful hypothesis will allow predictions based on reasoning. Reasoning can be broken down into two categories: deduction and induction. Most reasoning in science is done through induction.
Deduction involves determining a single fact from a general statement; it is only as accurate as the statement.
For example, if the teacher said she checks homework every Monday, she will check homework next Monday.
Deductions are intended to have reasoning that is valid. The reasoning in this argument is valid, because there is no way in which the reasons 1 and 2, could be true and the conclusion, 3, be false:
Reason 1: All humans are mortal.
Reason 2: Albert Einstein is a human.
Conclusion: Albert Einstein is mortal (Figure 1.6).

Induction involves determining a general statement that is very likely to be true, from several facts.
For example, if we have had a test every Tuesday for the past three months, we will have a test next Tuesday (and every Tuesday after that).
Induction contrasts strongly with deduction. Even in the best, or strongest, cases of induc- tion, the truth of the reason does not guarantee the truth of the conclusion. Instead, the conclusion of an inductive argument is very likely to be true; you cannot be fully sure it is true because you are making a prediction that has yet to happen.
A classic example of inductive reasoning comes from the philosopher David Hume:
Reason: The sun has risen in the east every morning up until now.
Conclusion: The sun will also rise in the east tomorrow.
Inductive reasoning involves reaching conclusions about unobserved things on the basis of what has been observed already. Inferences about the past from present evidence, such as in archaeology, are induction. Induction could also be across outer space, as in astronomy, where conclusions about the whole universe are drawn from the limited number of things we are able to observe.
A scientific experiment must have the following features:
a control, so variables that could affect the outcome are reduced
the variable being tested reflects the phenomenon being studied
the variable can be measured accurately, to avoid experimental error
the experiment must be reproducible.
An experiment is a test that is used to eliminate one or more of the possible hypotheses until one hypothesis remains. The experiment is a cornerstone in the scientific approach to gaining deeper knowledge about the physical world. Scientists use the principles of their hypothesis to make predictions, and then test them to see if their predictions are confirmed or rejected.
Scientific experiments involve controls, or subjects that are not tested during the investiga- tion. In this way, a scientist limits the factors, or variables that can cause the results of an investigation to differ. A variable is a factor that can change over the course of an experi- ment. Independent variables are factors whose values are controlled by the experimenter
www.ck12.org 12
to determine its relationship to an observed phenomenon (the dependent variable). Depen- dent variables change in response to the independent variable. Controlled variables are also important to identify in experiments. They are the variables that are kept constant to prevent them from influencing the effect of the independent variable on the dependent variable.
For example, if you were to measure the effect that different amounts of fertilizer have on plant growth, the independent variable would be the amount of fertilizer used (the changing factor of the experiment). The dependent variables would be the growth in height and/or mass of the plant (the factors that are influenced in the experiment). The controlled variables include the type of plant, the type of fertilizer, the amount of sunlight the plant gets, the size of the pots you use. The controlled variables are controlled by you, otherwise they would influence the dependent variable.
In summary:
The independent variable answers the question ”What do I change?”
The dependent variables answer the question ”What do I observe?”
The controlled variables answer the question ”What do I keep the same?”
In an old joke, a person claims that they are snapping their fingers ”to keep tigers away,” and justifies their behavior by saying, ”See, it works!” While this experiment does not falsify the hypothesis ”snapping your fingers keeps tigers away,” it does not support the hypothesis either, because not snapping your fingers will also keep tigers away. It also follows that not snapping your fingers will not cause tigers to suddenly appear (Figure 1.7).
To demonstrate a cause and effect hypothesis, an experiment must often show that, for example, a phenomenon occurs after a certain treatment is given to a subject, and that the phenomenon does not occur in the absence of the treatment.
One way of finding this out is to perform a controlled experiment. In a controlled exper- iment, two identical experiments are carried out side-by-side. In one of the experiments the independent variable being tested is used, in the other experiment, the control, or the independent variable is not used.
A controlled experiment generally compares the results obtained from an experimental sam- ple against a control sample. The control sample is almost identical to the experimental sample except for the one variable whose effect is being tested. A good example would be a drug trial. The sample or group receiving the drug would be the experimental group, and the group receiving the placebo would be the control. A placebo is a form of medicine that

Figure 1.7: Are tigers really scared of snapping fingers, or is it more likely they are just not found in your neighborhood? Considering which of the hypotheses is more likely to be true can help you arrive at a valid answer. This principle, called Occam’s razor states that the explanation for a phenomenon should make as few assumptions as possible. In this case, the hypothesis “there are no tigers in my neighborhood to begin with” is more likely, because it makes the least number of assumptions about the situation. (27)
www.ck12.org 14
does not contain the drug that is being tested.
Controlled experiments can be conducted when it is difficult to exactly control all the condi- tions in an experiment. In this case, the experiment begins by creating two or more sample groups that are similar in as many ways as possible, which means that both groups should respond in the same way if given the same treatment.
Once the groups have been formed, the experimenter tries to treat them identically except for the one variable that he or she wants to study (the independent variable). Usually neither the patients nor the doctor know which group receives the real drug, which serves to isolate the effects of the drug and allow the researchers to be sure the drug does work, and that the effects seen in the patients are not due to the patients believing they are getting better. This type of experiment is called a double blind experiment.
Controlled experiments can be carried out on many things other than people; some are even carried out in space! The wheat plants in Figure 1.8 are being grown in the International Space Station to study the effects of microgravity on plant growth. Researchers hope that one day enough plants could be grown during spaceflight to feed hungry astronauts and cosmonauts. The investigation also measured the amount of oxygen the plants can produce in the hope that plants could become a cheap and effective way to provide oxygen during space travel.

The term experiment usually means a controlled experiment, but sometimes controlled experiments are difficult or impossible to do. In this case researchers carry out natural
experiments. When scientists conduct a study in nature instead of the more controlled environment of a lab setting, they cannot control variables such as sunlight, temperature, or moisture. Natural experiments therefore depend on the scientist’s observations of the system under study rather than controlling just one or a few variables as happens in controlled experiments.
For a natural experiment, researchers attempt to collect data in such a way that the effects of all the variables can be determined, and where the effects of the variation remains fairly constant so that the effects of other factors can be determined. Natural experiments are a common research tool in areas of study where controlled experiments are difficult to carry out. Examples include: astronomy -the study of stars, planets, comets, galaxies and phenomena that originate outside Earth’s atmosphere, paleontology - the study of prehistoric life forms through the examination of fossils, and meteorology - the study of Earth’s atmosphere.
In astronomy it is impossible, when testing the hypothesis ”suns are collapsed clouds of hydrogen”, to start out with a giant cloud of hydrogen, and then carry out the experiment of waiting a few billion years for it to form a sun. However, by observing various clouds of hydrogen in various states of collapse, and other phenomena related to the hypothesis, such as the nebula shown in Figure 1.9, researchers can collect data they need to support (or maybe falsify) the hypothesis.
An early example of this type of experiment was the first verification in the 1600s that light does not travel from place to place instantaneously, but instead has a speed that can be measured. Observation of the appearance of the moons of Jupiter were slightly delayed when Jupiter was farther from Earth, as opposed to when Jupiter was closer to Earth. This phenomenon was used to demonstrate that the difference in the time of appearance of the moons was consistent with a measurable speed of light.
There are situations where it would be wrong or harmful to carry out an experiment. In these cases, scientists carry out a natural experiment, or an investigation without an experiment. For example, alcohol can cause developmental defects in fetuses, leading to mental and physical problems, through a condition called fetal alcohol syndrome.
Certain researchers want to study the effects of alcohol on fetal development, but it would be considered wrong or unethical to ask a group of pregnant women to drink alcohol to study its effects on their children. Instead, researchers carry out a natural experiment in which they study data that is gathered from mothers of children with fetal alcohol syndrome, or pregnant women who continue to drink alcohol during pregnancy. The researchers will try to reduce the number of variables in the study (such as the amount or type of alcohol consumed), which might affect their data. It is important to note that the researchers do not influence or encourage the consumption of alcohol; they collect this information from volunteers.
www.ck12.org 16

Figure 1.9: The Helix nebula, located about 700 light-years away in the constellation Aquar- ius, belongs to a class of objects called planetary nebulae. Planetary nebulae are the remains of stars that once looked a lot like our sun. When sun-like stars die, they puff out their outer gaseous layers. These layers are heated by the hot core of the dead star, called a white dwarf, and shine with infrared and visible colors. Scientists can study the birth and death of stars by analyzing the types of light that are emitted from nebulae. (50)
Field experiments are so named to distinguish them from lab experiments. Field experi- ments have the advantage that observations are made in a natural setting rather than in a human-made laboratory environment. However, like natural experiments, field experiments can get contaminated, and conditions like the weather are not easy to control. Experimental conditions can be controlled with more precision and certainty in the lab.
A prediction is a statement that tells what will happen under specific conditions. It can be expressed in the form: If A is true, then B will also be true. Predictions are based on confirmed hypotheses shown to be true or not proved to be false.
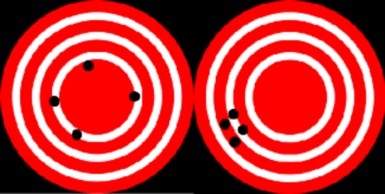
For researchers to be confident that their predictions will be useful and descriptive, their data must have as few errors as possible. Accuracy is the measure of how close a calculated or measured quantity is to its actual value. Accuracy is closely related to precision, also called reproducibility or repeatability. Reproducibility and repeatability of experiments are cornerstones of scientific methods. If no other researcher can reproduce or repeat the results of a certain study, then the results of the study will not be accepted as valid. Results are called valid only if they are both accurate and precise.
A useful tool to help explain the difference between accuracy and precision is a target, shown in Figure 1.10. In this analogy, repeated measurements are the arrows that are fired at a target. Accuracy describes the closeness of arrows to the bulls eye at the center. Arrows that hit closer to the bulls eye are more accurate. Arrows that are grouped together more tightly are more precise.
An error is a boundary on the precision and accuracy of the result of a measurement. Some errors are caused by unpredictable changes in the measuring devices (such as balances, rulers, or calipers), but other errors can be caused by reading a measuring device incorrectly or by using broken or malfunctioning equipment. Such errors can have an impact on the reliability of the experiment’s results; they affect the accuracy of measurements. For example, you use a balance to obtain the mass of a 100 gram block. Three measurements that you get are:
g, 92.0 g, and 91.8 g. The measurements are precise, as they are close together, but they are not accurate.
If the cause of the error can be identified, then it can usually be eliminated or minimized. Reducing the number of possible errors by careful measurement and using a large enough sample size to reduce the effect of errors will improve the reliability of your results.
www.ck12.org 18
Scientific theories are hypotheses which have stood up to repeated attempts at falsification and are thus supported by a great deal of data and evidence. Some well known biological theories include the theory of evolution by natural selection, the cell theory (the idea that all organisms are made of cells), and the germ theory of disease (the idea that certain microbes cause certain diseases). The scientific community holds that a greater amount of evidence supports these ideas than contradicts them, and so they are referred to as theories.
In every day use, people often use the word theory to describe a guess or an opinion. For example, “I have a theory as to why the light bulb is not working.” When used in this common way, “theory” does not have to be based on facts, it does not have to be based on a true description of reality. This usage of the word theory often leads to a misconception that can be best summed up by the phrase ”It’s not a fact, it’s only a theory.” In such everyday usage, the word is most similar to the term hypothesis.
Scientific theories are the equivalent of what in everyday speech we would refer to as facts. In principle, scientific theories are always subject to corrections or inclusion in another, wider theory. As a general rule for use of the term, theories tend to deal with broader sets of phenomena than do hypotheses, which usually deal with much more specific sets of phenomena or specific applications of a theory.
In time, a confirmed hypothesis may become part of a theory or may grow to become a theory itself. Scientific hypotheses may be mathematical models. Sometimes they can be statements, stating that some particular instance of the phenomenon under examination has some characteristic and causal explanations. These theories have the general form of univer- sal statements, stating that every instance of the phenomenon has a particular characteristic.
A hypothesis may predict the outcome of an experiment in a laboratory or the observation of a natural phenomenon. A hypothesis should also be falsifiable, and one cannot regard a hypothesis or a theory as scientific if it does not lend itself to being falsified, even in the future. To meet the “falsifiable” requirement, it must at least in principle be possible to make an observation that would disprove the hypothesis. A falsifiable hypothesis can greatly simplify the process of testing to determine whether the hypothesis can be proven to be false. Scientific methods rely heavily on the falsifiability of hypotheses by experimentation and observation in order to answer questions. Philosopher Karl Popper suggested that all scientific theories should be falsifiable; otherwise they could not be tested by experiment.
A scientific theory must meet the following requirements:
it must be consistent with pre-existing theory in that the pre-existing theory has been experimentally verified, though it may often show a pre-existing theory to be wrong in an exact sense
it must be supported by many strands of evidence rather than a single foundation, ensuring that it is probably a good approximation, if not totally correct.
Also, a theory is generally only taken seriously if it:
allows for changes to be made as new data are discovered, rather than claiming absolute certainty.
is the most straight forward explanation, and makes the fewest assumptions about a phenomenon (commonly called “passing the Occam’s razor test”).
This is true of such established theories as special relativity, general relativity, quantum mechanics, plate tectonics, and evolution. Theories considered scientific meet at least most, but ideally all, of these extra criteria.
In summary, to meet the status of a scientific theory, the theory must be falsifiable or testable. Examples of scientific theories in different areas of science include:
Astronomy: Big Bang Theory
Biology: Cell Theory; Theory of Evolution; Germ Theory of Disease
Chemistry: Atomic Theory; Kinetic Theory of Gases www.ck12.org 20
Physics: General Relativity; Special Relativity; Theory of Relativity; Quantum Field Theory
Earth Science: Giant Impact Theory; Plate Tectonics
The term theory is sometimes stretched to refer to theoretical speculation which is currently unverifiable. Examples are string theory and various theories of everything. String theory is a model of physics, which predicts the existence of many more dimensions in the universe than the four dimensions that current science understands (length, width, height, and space- time). A theory of everything is a hypothetical theory in physics that fully explains and links together all known physical phenomena.
For a scientific theory to be valid it must be verified experimentally. Many parts of the string theory are currently untestable due to the large amount of energy that would be needed to carry out the necessary experiments as well as the high cost of conducting them. Therefore string theory may not be tested in the foreseeable future. Some scientists have asked if it even deserves to be called a scientific theory because it is not falsifiable.
A superseded, or obsolete, scientific theory is a theory that was once commonly accepted, but for whatever reason is no longer considered the most complete description of reality by mainstream science. It can also mean a falsifiable theory which has been shown to be false. Giraffes, shown in Figure 1.11, are often used in the explanation of Lamarck’s superseded theory of evolution. In Lamarckism, a giraffe is able to lengthen its neck over its life time, for example by stretching to reach higher leaves. That giraffe will then have offspring with longer necks. The theory has been superseded by the understanding of natural selection on populations of organisms as the main means of evolution, not physical changes to a single organism over its lifetime.
Scientific laws are similar to scientific theories in that they are principles which can be used to predict the behavior of the natural world. Both scientific laws and scientific the- ories are typically well-supported by observations and/or experimental evidence. Usually scientific laws refer to rules for how nature will behave under certain conditions. Scientific theories are more overarching explanations of how nature works and why it exhibits certain characteristics.
A physical law or law of nature is a scientific generalization based on a sufficiently large number of empirical observations that it is taken as fully verified.

Figure 1.11: Superseded theories like Lamarck’s theory of evolution are theories that are now considered obsolete and have been replaced by newer theories that have more evidence to support them; in Lamarck’s case, his theory was replaced by Darwin’s theory of evolution and natural selection, which will be discussed in the chapter on Evolutionary Theory. (14)
Isaac Newton’s law of gravitation is a famous example of an established law that was later found not to be universal—it does not hold in experiments involving motion at speeds close to the speed of light or in close proximity of strong gravitational fields. Outside these conditions, Newton’s laws remain an excellent model of motion and gravity.
Scientists never claim absolute knowledge of nature or the behavior of the subject of the field of study. A scientific theory is always open to falsification, if new evidence is presented. Even the most basic and fundamental theories may turn out to be imperfect if new observations are inconsistent with them. Critical to this process is making every relevant part of research publicly available. This allows peer review of published results, and it also allows ongoing reviews, repetition of experiments and observations by many different researchers. Only by meeting these expectations can it be determined how reliable the experimental results are for possible use by others.
Scientific skepticism questions claims based on their scientific verifiability rather than accepting claims based on faith or anecdotes. Scientific skepticism uses critical thinking to analyze such claims and opposes claims which lack scientific evidence.
Science is based on the analysis of things that humans can observe either by themselves through their senses, or by using special equipment. Science therefore cannot explain anything about the natural world that is beyond what is observable by current means.
www.ck12.org 22
Supernatural things cannot be explained by scientific means.
Scientific investigations involve the collection of data through observation, the forma- tion and testing of hypotheses by experimentation, and analysis of the results that involves reasoning.
In a controlled experiment, two identical experiments are carried out side-by-side. In one of the experiments the independent variable being tested is used, in the other, the control, or the independent variable is not used.
Any useful hypothesis will allow predictions based on reasoning. Reasoning can be broken down into two categories: deduction and induction. Most reasoning in science is formed through induction.
A variable is a factor that can change over the course of an experiment. Independent variables are factors whose values are controlled by the experimenter to determine its relationship to an observed phenomenon (the dependent variable). Dependent variables change in response to the independent variable.
Scientific theories are hypotheses which have stood up to repeated attempts at falsifi- cation and are thus supported by much data and evidence.
What is the goal of science?
Distinguish between a hypothesis and a theory.
The makers of two types of plant fertilizers claim that their product grows plants the fastest and largest. Design an experiment that you could carry out to investigate the claims.
Identify how hypotheses and predictions are related.
What is the difference between the everyday term “theory” and the term “scientific theory?”
Identify two ways that scientists can test hypotheses.
Outline the difference between inductive and deductive reasoning.
What is the range of processes that scientists use to carry out a scientific investigation called?
To ensure that their results are not due to chance, scientists will usually carry out an experiment a number of times, a process called replication. A scientist has two types of plants and she wants to test which plant produces the most oxygen under sunny conditions outdoors. Devise a practical experimental approach, incorporating replication of the experiment.
In taking measurements, what is the difference between accuracy and precision?
Name two features that a hypothesis must have, to be called a scientific hypothesis.
Identify two features that a theory must have, to qualify as a scientific theory.
Give an example of a superseded theory.
Can a hypothesis take the form of a question? Explain your answer.
Why is it a good idea to try to reduce the chances of errors happening in an experiment?
http://www.project2061.org/publications/sfaa/online/chap1.htm#inquiry
http://www.nasa.gov/mission_pages/station/science/experiments/PESTO.html# applications
http://biology.plosjournals.org/perlserv/?request=index-html&issn=
http://www.estrellamountain.edu/faculty/farabee/biobk/diversity.htm
http://www.aaas.org/news/releases/2006/pdf/0219boardstatement.pdf
control Something that is not tested during the investigation.
controlled experiment Two identical experiments are carried out side-by-side; in one of the experiments the independent variable being tested is used, in the other experiment, the control, or the independent variable is not used.
controlled variables Variables that are kept constant to prevent influencing the effect of the independent variable on the dependent variable.
deduction Involves determining a single fact from a general statement.
dependent variable Changes in response to the independent variable.
experiment A test that is used to eliminate one or more of the possible hypotheses until one hypothesis remains.
hypothesis A suggested explanation based on evidence that can be tested by observation or experimentation.
independent variable Factor(s) whose values are controlled by the experimenter to de- termine its relationship to an observed phenomenon (the dependent variable).
induction Involves determining a general statement that is very likely to be true, from several facts.
www.ck12.org 24
observation The act of noting or detecting phenomenon through the senses. For example, noting that a room is dark is an observation made through sight.
Occam’s razor States that the explanation for a phenomenon should make as few assump- tions as possible.
phenomenon Is any occurrence that is observable.
scientific methods Based on gathering observable, empirical (produced by experiment or observation) and measurable evidence that is critically evaluated.
scientific skepticism Questions claims based on their scientific verifiability rather than accepting claims based on faith or anecdotes.
variable A factor that can change over the course of an experiment.
The Points to Consider section throughout this book is intended to have students think about material not yet presented. These points are intended to lead students into the next lesson or chapter.
Science is a particular way in which people examine and ask questions about the world. Can you think of other ways in which people examine and ask questions about the world?
Consider the importance of replication in an experiment and how replication of an experiment can affect results.
Scientists often disagree among themselves about scientific findings, and communicate such disagreement at science conferences, through science articles in magazines, or science papers and in scientific journals. Can you think of other ways in which scientists could communicate so that the public can get a better idea of what the “hot topics” in science are?
Outline the need for scientists to be able to share their ideas and findings with each other.
Identify the role of graphics in presenting results of an investigation.
Identify the role of peer review in the communication of ideas.
Examine how ethics are applied to communicating ideas and research.
Compare scientist to scientist communication to scientist to public communication.
Identify the benefits of studying science, even if you do not intend on becoming a scientist.
List three things that can influence scientific research.
Identify two ways that biotechnology has affected our lives.
The reliability of scientific knowledge comes partly from the objectivity of scientific meth- ods, and also from scientists discussing ideas with each other. In talking with each other, researchers must use more than just their scientific understanding of the world. They must also be able to convince a community of their peers of the correctness of their concepts and ideas.
A wide range of scientific literature is published and it is a format where scientific debates are properly carried out and reviewed. This includes scientific publications that report original research within a scientific field and can comprise of the following:
scientific articles published in scientific journals
books written by one or a small number of co-authors who are researchers
presentations at academic conferences, especially those organized by societies (for ex- ample, the American Association for the Advancement of Science)
government reports
scientific publications on the internet
books, technical reports, pamphlets, and working papers issued by individual re- searchers or research organizations
Scientific journals communicate and document the results of research carried out in univer- sities and various other research institutions. They are like a type of magazine that contains many articles which are written by different researchers about their ideas and discoveries. Most scientific journals cover a single scientific field and publish the research within that field; the research is normally expressed in the form of a scientific paper.
An academic conference is a conference for researchers (not always academics) to present and discuss their work. Together with scientific journals, conferences are an important channel for exchange of ideas between researchers. Generally, work is shared in the form
www.ck12.org 26
of visual posters or short presentations lasting about 10 to 30 minutes. These are usually followed by discussion. A researcher is presenting his work to his peers in Figure 1.12.
A scientific journal is a publication that reports new research, and sometimes contains general science news articles. Most journals are highly specialized for a particular field of research such as biochemistry, microbiology, or botany. However, some of the oldest journals such as Nature publish articles and scientific papers across a wide range of scientific fields. The journals shown in Figure 1.13 have a similar look and layout to science journals.
Scientific journals contain articles that have been peer reviewed in an attempt to ensure that articles meet the journal’s standards of quality, and scientific validity. A scientific journal is not usually read casually as you would read a magazine. Some of the content can be very dense and detailed.
The publication of the results of research is an essential part of the scientific process. The researcher who has written the paper must give enough details about their experiments so that an independent researcher could repeat the experiment to verify the results.
The significance of these different parts of scientific literature differs between science disci- plines and has changed over time. Peer-reviewed journal articles remain the most common publication type and have the highest level of trust. However, journals vary enormously in
their prestige and importance, and the value of a published article depends on the journal, review process and the degree that it is referenced by other scientists.
Some well known and well respected science and medical journals include:
Science
Nature
Proceedings of the National Academy of Sciences of the United States of America (PNAS)
Public Library of Science (PLoS)
Cell
Journal of the American Medical Association (JAMA)
The Lancet
Journal of Theoretical Biology

New research is usually written up in the form of a scientific article, which often appear in journals. A scientific article has a standardized structure, which varies only slightly between the different sciences. This format can also be used for your lab reports as part of this class.
www.ck12.org 28
It is not really the format of the article that is important, but what lies behind it or the content. However, several key format requirements need to be met by every science article:
The title should be short and indicate the contents of the article.
The names of all authors that were involved in the research should be given. Where the authors work or study should also be listed.
The first section is normally an abstract: a one-paragraph summary of the work. The abstract is intended to serve as a quick guide for the reader as to the content of the article.
The format should be able to be stored in a library so that scientists years later will be able to recover any document in order to study and assess it
The content of the study should be presented in the context of previous scientific inves- tigations, by citing related documents in the existing literature. This is usually in a section called an introduction.
Observations that were made, and measurements that were taken are described in a section usually called Materials and Methods. The experiments should be described in such a way that other scientists in the same or related fields can repeat the experiments and observations and know whether he or she gets the same results. This is called reproducibility.
Similarly, the results of the investigation are given in a section called, results. Data should be presented in tabular or graphic form (images, charts, graphs, photos, or diagrams, shown in Figure 1.14. Graphics should have a caption to explain what they are showing.
Interpretation of the meaning of the results is usually addressed in a discussion and/or conclusion section. The conclusions drawn should be based on previous studies and/or new scientific results. They should also be written in a way such that any reader with knowledge of the field can follow the argument and confirm that the conclusions are sound.
Finally, a references or literature cited section lists the sources cited by the authors in the format required by the journal.
The reliability of information is dependent on whether the information appears in a primary source, secondary source, or a tertiary source.
Most research studies are first published in a scientific journal, which are referred to as
primary sources. Technical reports, for minor research results are also primary sources.
Secondary sources include articles in review journals (collections of recent research articles on a topic). Review journals are usually published to highlight advances and new lines of research in specific areas, such as human genetics, specific medical disorders (such as heart disease), neurology (the study of the nervous system) or malacology, (the study of snails and other mollusks). Large projects, broad arguments, or a mix of different types of articles may
appear in a book. Review journals and books are referred to as secondary sources. Tertiary sources might include encyclopedias and news articles which are generally written for the public to read.
Scientists are expected to report their work truthfully and honestly. They are also expected to have their work reviewed by fellow scientists. This process is called peer review.
Peer review is a process of opening a scientist’s research or ideas (in the form of a scientific paper) to examination by other scientists who are experts in the same field. The peer review process aims to make authors meet the standards of their area of study, and to meet the expected standards of science in general. Publications that have not undergone peer review are likely to be regarded with suspicion by scholars and professionals in many fields. However, even peer reviewed journals can contain errors.
A reason for the need for peer review is that it is rare for an individual author or research team to spot every mistake or flaw in a complicated piece of work. The review process provides an opportunity for improvement because a person with special expertise or experience reads the research paper before it is published. Typically, for publication in a science journal, it is also a requirement that the research is new and useful. Since reviewers are normally selected from
www.ck12.org 30
experts in the areas of science covered by the article, the process of peer review is considered vital to establishing a reliable body of research and knowledge. Therefore, showing work to other scientists increases the likelihood that weaknesses will be found and corrected.
The process of peer review is not designed to detect fraud. As a result, there is usually a large scandal when a researcher and author of a science paper is found to have falsified the research in an article, as many other researchers may have relied upon their original research for their own work or the researcher could have received grant money based on falsified research. Peer review of scientific work assumes that the article reviewed has been honestly written. Usually reviewers do not have full access to the data from which the paper has been written, so they trust that the author is being truthful and honest.
It is important for the researcher to remain neutral or objective when conducting scientific research. A bias is a position for favoring one particular point of view over another, and it is usually based on preconceived ideas about a situation. The inability of a human being to remain completely objective is the source of such bias in research. Nevertheless, a researcher or their study is generally said to be biased only if the researcher’s judgment is influenced by the biases they hold, which could influence their research results.
For example, you want to test whether your dog, Frankie, prefers his regular food or the super expensive brand dog food that you have just bought on sale. You would put each food in a bowl and offer both foods to Frankie at his meal time. However, you secretly hope he prefers his regular food because it is half the price of the more expensive food and you can buy it in the store down the road. Frankie takes a couple of mouthfuls of his regular food, but gobbles up all of the expensive food. You may think, “Well, he did eat some of regular food, so he still likes it,” when in fact Frankie clearly preferred the expensive brand. You buy the regular food anyhow. Whether you like it or not, you are biased toward the regular dog food.
This example above is greatly simplified, but, illustrates how personal opinions may influence an investigation.
Another type of bias, called a systematic bias is introduced from a flaw in measurements. For example, an incorrectly calibrated thermostat may consistently read several degrees hotter or colder than actual temperature. As a consequence, systematic bias commonly leads to systematic errors in the results of an investigation. Peer review can usually detect systematic biases in a research study.
A conflict of interest is a situation in which a researcher has professional or personal interests that are at odds with each other. For example, a researcher is about to investigate a new headache medicine from a drug company called Tinneas. The researcher carries out experiments and finds that the medicine works very well. End of story, right? Not exactly.
Later it is discovered that the researcher owns Tinneas stock. This means he owns part of the company. Even if everything was done correctly during the experiment, and the drug really does work, this researcher has a conflict of interest. As an owner of the company, he will earn money if the drug works, but will lose money if the drug does not work. Therefore, any scientist that may have a reason to favor one particular result from an investigation should not be involved in that investigation.
Competing interests can make it difficult for a person to carry out his or her duties without bias. A conflict of interest exists even if no wrong has been done, or nothing results from it. A conflict of interest can affect the public confidence in the person, a profession, or company.
When presenting their research to others, an ethical scientist would not falsify results, lie about their results, or plagiarize (steal other peoples ideas or work).
Scientific misconduct is the violation of these standard codes of scholarly conduct and ethical behavior in professional scientific research. Scientific misconduct may take place simply out of reputation. For example, academic scientists are often under enormous pressure to produce publications in peer reviewed journals. Alternatively, there may be commercial or political motivations where the financial or political success of a project depends on publishing evidence of a procedure working or not working. The consequences of scientific misconduct can be severe at a personal and professional level for the people involved. In addition, there are public health concerns attached to the promotion of medical or other procedures that are founded on doubtful research results.
Some instances of scientific fraud and scientific misconduct have gone through review and were detected only after other groups tried and failed to replicate the published results. An example is the case of physicist Jan Hendrik Schön, in which a total of fifteen papers on microelectronics and nanotechnology were accepted for publication in the top ranked journals, Nature and Science, following the usual peer review process. All fifteen were found to be fraudulent and were then withdrawn. The fraud was found, not by the peer review process, but by other research groups who tried and failed to reproduce the results of the paper.
www.ck12.org 32
Likewise, biomedical scientist Hwang Woo-Suk, rose to fame after claiming a series of break- throughs in the field of stem cell research. He was once considered one of the pioneering experts in the field of stem cell research, because of his success in creating cloned human embryonic stem cells. However, his two most famous research articles on the cloning ex- periments were found to contain large amounts of fabricated data. Hwang’s papers were retracted (withdrawn from publication), he lost his job at the university where he worked, and also lost his research funding.
Science has become such a part of modern life that it is necessary to communicate the achievements, news, and ambitions of scientists to a wider audience. Scientists need to be able to tell each other and the public about their research and the results of their research. These two groups make up two very different audiences for scientists, however. The first audience is made up of their peers-fellow scientists who have an advanced understand of the technical language and procedures that are involved in scientific investigations. The second audience is made up of members of the public who may or may not understand or know about their research. For example, the following passage is a summary of a paper that appears in the Public Library of Science (PLoS), an online science journal:
A systematic analysis of Alzheimer disease amyloid peptide variants in Drosophila brain demonstrates that their predicted propensity to form protofibrillar aggregates correlates best with toxicity.
Biologists would have no problem understanding the language in this paragraph. However, to a person who is not familiar with this type of science, it may be interpreted as gibberish. In this, lies the challenge for scientists to communicate their research in a way that the general public can understand.
The results of the study could be written in the following way so that a general reader could follow what the researchers meant:
Studies of a particular type of brain protein, called amyloid peptides, have shown that they can sometimes change into a defective form that resembles sticky clumps. These clumps may become toxic and contribute to Alzheimer’s disease, a wasting disease of the brain. Researchers are examining these proteins to find out what exactly causes them to form such clumps. The studies were carried out on fruit flies, which are commonly used as animal models for genetic and biochemical studies of humans.
Many scientists do a good job of presenting their work in an accessible way on the Internet. Scientists and science journalists write news articles that explain the research in everyday
language, and can show how the research relates to the reader and to their environment. For example, who would want to read an article that only talked about research that is taking place at the South Pole? An article packed with numbers, units, and percentage rates would be pretty boring to read if it were not related to other areas such the environment, people, animals, or the climate. Also, presenting such academic subjects in a readable and engaging way, allows people to understand what research is being done and why. Such general presentation of science appeals to people because it allows the reader to relate the subject to their life and experiences. For example, both the National Science Foundation (NSF) U.S Antarctic Program and the International Polar Year (IPY) 2007-2008 have websites that explain the types of research that is going on in Antarctica and the Arctic. An NSF research vessel that is taking part in the IPY 2007-2008 is shown in Figure 1.15.

A science magazine is a publication with news, opinions and reports about science and is written for a non-expert audience. Compare this to a scientific journal, which is written by and for scientific researchers. Science magazines are read by non-scientists and scientists who want accessible information on fields outside their specialization. Articles in science magazines are sometimes republished or summarized by the general press, in newspapers, online news sites, and blogs among other media forms.
Science magazines such as New Scientist, shown in Figure 1.16, and Scientific American, have non-technical summaries of popular areas of research, notable discoveries, and scientific advancements in different fields of research. Science books engage the interest of many more people. So, too, do science websites and science television programming add more images
www.ck12.org 34
and illustrations that help tell a story. In this way, more people can become more aware of how science effects their lives and become better informed about science subjects.

Figure 1.16: Cover of New Scientist magazine. (6)
You may have already heard the term scientific consensus being used when the subject of global warming is talked about in the news. Scientific consensus is the collective judgment, position, and opinion of a community of scientists in a particular field of science, at a particular time. Scientific consensus is not, by itself, a scientific argument, and is not part of the “scientific method”. But the topic for which a consensus exists may itself be based on both scientific arguments and scientific methods.
Consensus is normally carried out by scientists talking to each other and sharing their ideas and findings. Scientists can accomplish consensus by giving talks or presentations at confer- ences, or by publishing their ideas and findings for other scientists to read. This can lead to a situation where those within the field of science can recognize a consensus when it exists, but communicating that to others, such as non-scientists or the public, can be difficult. Some- times, scientific institutes release statements that are meant to communicate a summary of the science from the inside to the outside. In cases where there is little controversy regarding the subject under study, laying out what the consensus is about can be straightforward.
Nevertheless, scientific consensus may be used in popular or political debate on subjects such as evolution or climate change that are controversial within the public sphere, but are not controversial within the scientific community.
Biology literally means ”the study of life,” and it is also a science that is very close to our everyday lives. Biology is a very broad field, covering the intricate workings of chemical processes inside our cells, to the more broad concepts of ecosystems and global climate change. Biologists study minute details of the human brain, the make up of our genes, and even the functioning of our reproductive system. For example, biologists recently finished decoding the human genome, the sequence of deoxyribonucleic acid (DNA) bases that may determine much of our abilities and predispositions for certain illnesses and can also play a major role in many court cases. For example, criminals have been caught, victims identified, and wrongly imprisoned people have been freed based on DNA evidence.
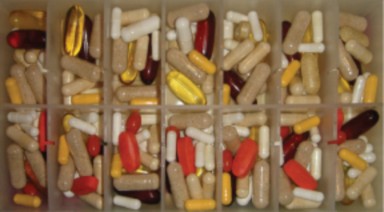
We are blitzed with headlines about possible health risks from certain foods as well as possible benefits of eating other foods. Commercials try to sell us the latest “miracle” pill for easy, fast weight loss. Many people are turning to herbal remedies to ease arthritis pain, improve memory, as well as improve their mood. Other people may choose the conventional medicines that can be bought at the pharmacist. It is important to know the effects such supplements, such as the ones shown in Figure 1.17, and medicines can have on the body.
Can a biology book give you the answers to these everyday questions? No, but it will enable you learn how to sift through the biases of investigators, the press, and others in a quest to critically evaluate the question. To be honest, five years after you are finished with this biology book, it is doubtful you would remember all the details of metabolism. However, you will have a better idea about where to look for the answer. Knowing about the process of science will also allow you to make a more informed decision. Will you be a scientist? Yes, in a way. You may not be formally trained as a scientist, but you will be able to think
www.ck12.org 36
critically, solve problems, have some idea about what science can and cannot do, as well as an understanding of the role of biology in your everyday life.
So why should you study biology? Because you are surrounded by it every day! It is about what happens in your brain as your read the words on this page and about how hippopotamuses know to come up to the surface to breath even while sleeping. Biology is about why a person with hook worms doesn’t sneeze as much and about why Velcro works. From understanding the benefits of the vitamin-enriched milk or juice you that have at breakfast, to discerning commercials that promise smoother thighs or a fuller head of hair, or snack foods that announce they are the “health busy livelier option for your,” you cannot be fully informed about such claims unless you understand the science behind them, or can think like a scientist to analyze them. For example, you would need to know the types of fats you need to get from your food to know why eating salmon, shown in Figure 7 1.18, or other foods such as flax seeds and kiwifruit may be good for your health.

You may also become a stronger advocate for your community. For example, if a tree planting initiative has begun in your neighborhood, you can investigate the plan for your area and find out what you can do. You could then explain what the program is about to your friends and family.
Or, perhaps a city park has fallen into disrepair, and city officials are looking for feedback from the public about what to do with it. You could use scientific thinking to analyze the issue and options, and develop some solutions.
What exactly makes a person a “scientist” and what is their role in society? First, we should start with what scientists are not. They are not crazed geniuses with bad hair and a fondness for hysterical laughter, as Figure a 1.19 might suggest. Although they may not be on the cutting edge of fashion, they are regular people. They went to school like you, they studied math, reading, and science like you, and they probably exhibited at science fairs, just like the students in Figure b 1.19.

Being a scientist does not require you to learn everything in this book or any other science book by heart, but understanding the important concepts really helps. Instead, being a scientist begins by thinking like a scientist. Scientists are curious about how the world works; they have many questions and go about answering those questions using the scientific methods, which we discussed in the Nature of Science lesson.
If you are fascinated by how things work and why they work a certain way, you too could become a scientist! Research scientists are the people that do the investigations and make the discoveries that you read or hear about. To work as a research scientist, a person usually needs an advanced degree in science. An advanced degree is obtained by attending graduate school after getting a Bachelor of Science, Engineering, or Arts degree. A Bachelor degree normally takes four years to complete; graduate degrees usually take two years for a Masters degree and four or more years to complete a Doctorate degree.
Scientific research offers much more to a person than just discovering new things. Researchers have the opportunity to meet with other people (scientists and non-scientists) who care about the same subjects that the scientists research such as cancer research, marine ecology, or human nutrition. Many researchers also teach students who will become the next generation
www.ck12.org 38
of scientists. Scientists have many opportunities to work with different people, explore new fields, and broaden their expertise.
Scientists are part of a community that is based on ideals of trust and freedom, and their work can have a direct effect on society. As a result, the public usually has an interest in the results of research that will directly affect them. Therefore it is important that you can understand the meaning of a science story when you read it, see it, or hear about it and become an engaged and active member of the public when making decisions involving science.
Conducting science requires part human creativity and part scientific skepticism. Researchers make new observations and develop new ideas with the aim of describing the world more accurately or completely. These observations and ideas are often based on existing theories and observations that were made by earlier scientists.
For example, the history of molecular biology, the study of molecules that make up living things, is a good example of how scientific knowledge builds on earlier knowledge.
Researchers from chemistry and physics were involved in the early investigations to discover what was responsible for heredity. Scientists in the late 19th and early 20th century knew that organisms inherited certain characteristics such as hair color from their parents. What we now call ”genes” were then called “units of heredity.” Scientists did not know exactly how these heredity units were inherited or what they were made of, however. Following the development of the Mendelian theory of heredity in the 1910s and the development of atomic theory and quantum mechanics in the 1920s, such explanations seemed within reach. Researchers from chemistry and physics turned their attention to this biological question. Still, in the 1930s and 1940s it was not clear which, if any, area of research would be most successful.
In 1940, geneticists George Beadle and Edward Tatum demonstrated a relationship between genes and proteins. In 1944, physician and researcher Oswald Avery further elaborated on that finding by demonstrating that genes are made up of DNA. In 1952, geneticist Alfred Hershey and lab assistant Martha Chase confirmed that the genetic material of a virus that infects bacteria is made up of DNA. And in 1953, biologist James Watson and biophysicist Francis Crick, with the help of X-ray crystallographer Rosalind Franklin, worked out the three dimensional structure of DNA and built a model of the double helix structure of the molecule.
There have been many additional discoveries about DNA and heredity since then, which you will learn more about in the Molecular Genetics and Biotechnology chapters.
To nonscientists, the competition, frustration, cooperation, and disagreement between re- search scientists can seem disorganized. Scientific knowledge develops from humans trying to figure things out. Scientific research and discoveries are carried out by people—people who have virtues, values, shortcomings, and limitations—just like everyone else. As a result, science and research can be influenced by the values of the society in which the research is carried out. How do such values influence research?
This question is of interest to more than just the scientific community. Science is becoming a larger part of everyone’s life, from developing more effective medicines to designing innovative sustainable air conditioning systems that are modeled after the self-cooling nests of termites. The public has become more interested in learning more about the areas of science that affect everyday life. As a result, scientists have become more accountable to a society that expects to benefit from their work.
It costs money to carry out scientific studies. Things such as the cost of equipment, trans- portation, rent, and salaries for the people carrying out the research all need to be considered before a study can start. The systems of financial support for scientists and their work have been important influences of the type of research and the pace of how that research is con- ducted. Today, funding for research comes from many different sources, some of which include:
Government, for example, through the National Institutes of Health (NIH), Center for Disease Control and Prevention (CDC), and the Food and Drug Administration (FDA)
Military funding (such as through the Department of Defense)
Corporate sponsorship
Non-profit organizations, such as the American Cancer Society, Stroke Awareness For Everyone, Inc. (SAFE)
Private donors
When the economy of a country slows down, the amount of money available for funding research is usually reduced, because both governments and businesses try to save money by cutting out on non-essential expenses.
Many pharmaceutical companies are heavily invested in research and development, on which they spend many millions of dollars every year. The companies aim to research and develop drugs that can be marketed and sold to treat certain illnesses, such as diabetes, cancer, or high blood pressure. Areas of research in which the companies do not see any hope of a return on their huge investments are not likely to be studied.
For example, two researchers, Evangelos Michelakis and Steven Archer of the University of Alberta, Canada, recently reported that a drug that has been used for in the treatment of rare metabolic disorders could be an effective drug for the treatment of several forms of
www.ck12.org 40
cancer. Dichloroacetic acid, (DCA), is a chemical compound that appears to change the way cancer cells get energy, without affecting the function of normal cells. The researchers found that DCA killed cancer cells that were grown in the lab and reduced the size of tumors in rats.
However, DCA is non-patentable as a compound. A patent is a set of rights granted to a person or company (the patentee) for a certain period of time which allows the patentee the exclusive right to make, use, sell, or offer to sell the patented item. Because DCA cannot currently be patented, concerns are raised that without the financial security a patent would ensure, the financial incentive for the pharmaceutical industry to get involved in DCA-cancer research would be reduced, and therefore clinical trials of DCA may not be funded.
But, other sources of funding exist; previous studies of DCA have been funded by govern- ment organizations such as the National Institutes of Health (NIH), the Food and Drug Administration (FDA), the Canadian Institutes of Health Research and by private charities such as the Muscular Dystrophy Association. Recognizing the possible challenges to funding, Dr. Michelakis’s lab took the unusual step of directly asking for online donations to fund the research. After six months, his lab had raised over $800,000, which was enough to fund a small clinical study. Dr. Michelakis and Dr. Archer have nonetheless applied for a patent on the use of DCA in the treatment of cancer.
Funding for research can also be influenced by the public and by social issues. An intense amount of public interest was raised by the DCA study. The story received much media attention in early 2007. As a result, the American Cancer Society and other medical or- ganizations received a large volume of public interest and questions regarding DCA. A few months later, the Department of Medicine of Alberta University reported that after the trial funding was secured, both the Alberta local ethics committee and Health Canada approved the first DCA Clinical Trial in Cancer.
Government funding of research can be indirectly influenced by the public. Funding priorities for specific research can be influenced by the ethical beliefs or reservations of elected public officials, or influenced by the public during constitutional amendment elections. Celebrities, often campaign to bring public attention to issues that are important to them. For example, Lance Armstrong, in Figure 1.20, talks publicly about his experiences as a former cancer patient to help raise awareness about cancer research and the importance of funding for clinical trials.
Ethics, also called moral philosophy, is the discipline concerned with what is morally good and bad, right and wrong. The term is also applied to any system or theory of moral values or principles. Personal ethics is the moral code that a person adheres to, while social ethics includes the moral theory that is applied to groups. Bioethics is the social ethics of biology and medicine; it deals with the ethical implications of biological research and applications,

especially in medicine. Bioethicists are concerned with the ethical questions that arise in the relationships among biology, biotechnology, medicine, politics, law, and philosophy.
While scientific research has produced social benefits, it has also posed some troubling ethical questions. For example, when is it okay to test an experimental cancer drug on people? Developing a new drug takes a long time, maybe as much as 10 years, or more. There are many rules and regulations that drug researchers need to stick to while developing drugs to treat specific illnesses.
Generally, drugs cannot be tested on people until researchers have evidence that the drug does the job that they claim it does (in this case kills cancer cells), but also that the drug will not make patients more ill or cause death. However, if the drug has tested successfully in earlier experiments, and scientists are quite confident that the drug does help kill off cancer cells, is it ethical to allow patients with terminal cancer, who have no other treatment options, to try the experimental drug?
With new challenges in public health and health policy, and with advances in biotechnology, bioethics is a fast-growing academic and professional area of inquiry. Some recent bioethical debates also include:
Refusal of medical treatment The choice of a patient to refuse certain life-saving med- ical procedures such as a blood transfusion, or refusal by a parent or guardian for medical treatment for the patient.
Euthanasia The choice by a terminally ill person to have medical assistance in dying.
Stem cell research Research involving stem cells, which can be harvested from human embryos.
www.ck12.org 42
Animal cloning The ability and usefulness of scientists cloning animals for various needs, such as vaccine development, tissues for transplant into humans such as heart valve, and increased food production. Dolly the sheep, probably the most famous animal clone to date, is shown in Figure 1.21.

Because research may have a great effect on the wellbeing of individual people and society in general, scientists are required to behave ethically. Scientists who conduct themselves ethi- cally treat people (called subjects) who are involved in their research respectfully. Subjects are not allowed to be exploited deliberately, exposed to harm, or forced to do something they do not agree to.
A lot of popular science articles come from sources whose aim is to provide a certain amount of entertainment to the reader or viewer. Many popular science articles will examine how a phenomenon relates to people and to their environment. Nevertheless, there is a ten- dency in the popular media to dilute scientific debates into two sides, rather than cover the complexities and nuances of an issue.
Even well-intentioned scientists can sometimes unintentionally create truth-distorting media
firestorms because of journalists’ difficulty in remaining critical and balanced, the media’s interest in controversy, and the general tendency of science reporting to focus on apparent ”groundbreaking findings” rather than on the larger context of a research field. Sometimes scientists will seek to exploit the power of the media. When scientific results are released with great fanfare and limited peer review, the media often requires skepticism and further investigation by skilled journalists and the general public.
The dichloroacetic acid (DCA) story, discussed earlier in this lesson, is an example of what can go wrong when a scientific discovery grasps the public’s attention.
An intense amount of public interest was raised by the study and the story received much media attention. As a result, the American Cancer Society and other medical organizations received a large volume of public interest and questions about the “miracle cure,” DCA.
One of the first stories about the findings contained the headline: “Cheap, ’safe’ drug kills most cancers”
The article did explain that the studies were only carried out on cancer cells grown in the lab and in rats. However, the headline may have given some readers the impression that human testing of DCA was complete. People were wildly interested in this new “cure” to cancer. This prompted the American Cancer Society and other organizations to issue reports that reminded people that although the study results were promising, no formal clinical trials in humans with cancer had yet been carried out. They stressed the need for caution in interpreting the early results. Doctors warned of possible problems if people attempted to try DCA outside a controlled clinical trial. The media received some criticism for the sensation that arose due to their coverage of the discovery.
Therefore, it is important to remember as a member of the public that some popular science news articles can be misleading. A reader can misinterpret the information, especially if the information has a emotional affect on the reader. Also, some articles are written by people who have limited understanding of the subject they are interpreting and can be produced by people who want to promote a particular point of view. Unfortunately, it can be difficult for the non-expert to identify misleading popular science. Sometimes, results are presented in the media without a context, or are exaggerated. Popular science may blur the boundaries between formal science and sensationalism. It is best to analyze such information with skepticism as you would if you were to make an observation in an investigation, and look at the whole context of an issue, rather than just the focus of a particular news item.
For example, in early 1999 West Nile virus, a virus most commonly found in Egypt, was accidentally introduced to New York. Although infection by the virus causes mostly mild or no symptoms in people, in rare instances, West Nile virus can cause inflammation of the brain. The illness, called West Nile Fever, spread across the continent from east to west, carried by infected birds. Mosquitoes spread the disease to mammals. Mosquito larvae (young) are shown in Figure 1.22.
There was intense media coverage about the spread of this disease across the United States, www.ck12.org 44
and much talk about what this meant for everyone. News coverage of West Nile Fever tended to focus on the serious form of the disease, West Nile Encephalitis, which can cause harmful illness and death. The fact that there is no vaccine for the disease was also emphasized.

However, it is worthwhile considering that until October 2007 there had been a total of 26, 997 confirmed cases of West Nile virus infection, and 1,038 confirmed deaths from the disease. Compare this to the estimated 15 to 60 million people in the United States who are infected with the flu virus every year, and the estimated 36,000 people who die every year from flu complications.
So the next time you are shocked or horrified by a seemingly gloomy forecast in the media, consider how the issue fits into the bigger story.
Biotechnology is technology based on biology; it involves the use of organisms or biological processes and can be especially used in agriculture, food science, and medicine. It is the application of biological knowledge to develop tools and products that allow us to control and adapt to our environment.
Biotechnology has effected society and in a number of ways. Although it has been used for centuries in traditional production processes, such as animal breeding shown in Fig- ure 1.23, crop growing, and wine making, modern biotechnology is a recent field of science. Bioengineering is the science upon which all biotechnological applications are based. New de-
velopments and new approaches are developing at a very fast pace. Biotechnology combines scientific fields such as genetics, molecular biology, biochemistry, and cell biology.

The field of modern biotechnology is thought to have largely begun in 1980, when the United States Supreme Court ruled that a genetically-modified microorganism could be patented. Indian-born researcher, Ananda Chakrabarty, had developed a bacterium that was able to break down crude oil, which he proposed to use in treating oil spills.
Biotechnology has applications in four major industrial areas, including health care, crop production and agriculture, non-food uses of crops such as biofuels, and environmental uses. One application of biotechnology uses organisms to produce things such as nutritional sup- plements like vitamins or amino acids, and milk products like cheese, kefir, and yogurt. Biotechnology is also used to recycle, treat waste, and clean up sites contaminated by indus- trial waste. The use of microorganisms to clean up contaminated sites such as an oil spill is called bioremediation.
Medical applications of biotechnology include designing organisms to produce medicines such as antibiotics, or other chemicals. Medical applications for people also include gene therapy
www.ck12.org 46
which could be used to treat a person who has a genetic disorder such as cystic fibrosis.
An example of an agricultural application is designing plants to grow under specific envi- ronmental conditions or in the presence (or absence) of certain chemicals, such as the cress shown in Figure 1.24. The cress plant has been genetically modified to turn red only in the presence of nitrogen dioxide, a chemical that is released by landmines and other unexploded bombs. Researchers at the Danish biotechnology company that developed the plant hope that the seeds can be spread over former battleground areas where they will grow and mark the sites of the explosives, thus speeding up the land mine removal process.

Figure 1.24: This thale cress Arabidopsis thaliana has been genetically modified to turn red only in the presence of nitrogen dioxide, a chemical marker for landmines or other unexploded bombs. Researchers hope that the cress seeds can be spread over former battleground areas, where they will grow and mark the sites of explosives, thus lessening the risk to the people and animals who live in those areas and work to remove the explosives. (45)
Another hope is that biotechnology might produce more environmentally friendly solutions than traditional industrial agriculture. An example of this is the engineering of a plant to express a pesticide, which cuts out the need to apply pesticides to the plants. The corn plants in Figure 1.25 have been genetically modified (changed) to produce a toxin that comes from a naturally occurring soil bacterium called Bacillus thuringiensis. The Bt toxin kills the pests that eat and destroy corn crops. Whether or not biotechnology products such as this are more environmentally friendly in the long run is a hot topic of debate.
Bioinformatics is an interdisciplinary field which helps solve biological problems using com- puters. Lots of information is gathered from the mapping of DNA sequences and other related types of research. Bioinformatics allows scientists to gather this information, share it and to use it. It also speeds up the process of analyzing data the scientists have collected. The field may also be called computational biology. Bioinformatics plays a key role in various areas, and it is a key part of the biotechnology and the pharmaceutical industries.

Figure 1.25: People looking at a sign that explains what the genetically modified corn does. In an effort to reduce corn stem-borer infestations, corporate and public researchers came together to develop genetically modified corn varieties suitable for Kenya. The corn plants contain a gene (Bt gene) from a naturally occurring bacterium called Bacillus thuringiensis. The Bt gene causes the corn plants to make Bt toxin which kills the pests that feed on the plants. (47)
www.ck12.org 48
Psychologists David Patterson and Hunter Hoffman of the University of Washington in Seattle developed a virtual world computer game they called “Snow World” shown in Figure 1.26, in an effort to reduce the pain experienced by patients undergoing burn treatment and other medical procedures. They found that people who became fully engaged in the virtual reality snow world reported 60 percent less pain. This technology offers a promising new way to manage pain. The researchers say that an interactive digital world may distract us from reality because our minds focus on just a few things at once.

The reliability of scientific knowledge comes partly from the objectivity of scientific methods, and also from scientists discussing ideas with each other. In talking with each other, researchers must use more than just their scientific understanding of the world. They must also be able to convince other scientists of the accuracy of their ideas.
Graphics help to illustrate ideas that would otherwise be too confusing to describe in words only.
The peer review process aims to make authors meet the standards of their area of study, and to meet the expected standards of science in general.
Ethics is the discipline concerned with what is morally good and bad, right and wrong. Bioethics is the social ethics of biology and medicine; it deals with the ethical im- plications of biological research and applications, especially in medicine. Bioethicists are concerned with the ethical questions that arise in the relationships among biology, biotechnology, medicine, politics, law, and philosophy.
Scientists need to be able to tell each other and the public about their research and the results of their research. These two groups make up two very different audiences for scientists. Presenting academic subjects in a readable and engaging way, allows the general pubic to understand what research is being done and why. Presentation of generally written science appeals to people because it allows the reader to relate the subject to their life and experiences.
You cannot be fully informed about the scientific issues you read about unless you understand the science behind the issues, or have the ability to think like a scientist to analyze them.
The cost of equipment, transportation, rent, and salaries for the people carrying out the research all need to be considered before a scientific study can start. The systems of financial support for scientists and their work have been important influences of the type of research and the pace of research. Today, funding for research comes from many different sources.
Biotechnology is the application of biological knowledge to develop tools and products that allow us to control and adapt to our environment.
What is bias in scientific terms and how is it relevant to science?
Who do you think the ethical rules about scientific research are aimed toward? Who do they protect?
Investigate a science-based societal issue that affects your town, city, or state. Research literature and news reports about the issue, analyzing the data, and examine what an individual person, the community, the local government, or federal government could do about this issue. Present your finding in the form of a poster or computer slide presentation to your class.
Find a science article that you believe could be improved upon by adding a graph, a picture, or a drawing. Rewrite the article in your own words, and present it to your class, along with your added graphics.
How has biotechnology affected modern life?
Science and biotechnology are pursued for different purposes. Do you agree with this statement? Explain your answer.
Identify an ethical issue that is raised by biotechnology.
Identify an ethical issue that is raised by media coverage of science.
Why is it a good idea to study science even if you do not want to become a career scientist?
What are three sources of funding for scientific research?
How might ethics affect funding for scientific research? www.ck12.org 50
http://response.restoration.noaa.gov/faq_topic.php?faq_topic_id=1
http://www.ctv.ca/servlet/ArticleNews/story/CTVNews/20070120/DCA_feature_ 070121/20070122?hub=Health
http://www.newscientist.com/article/mg19325890.200-no-wonder-drug.html
http://publications.nigms.nih.gov/biobeat/gallery/index.html
http://publications.nigms.nih.gov/findings/sept05/bedside_sept05.html
abstract A brief, usually one-paragraph, summary of the work.
academic conference A conference for researchers (not always academics) to present and discuss their work.
animal cloning The ability and usefulness of scientists cloning animals for various needs, such as vaccine development, tissues for transplant into humans such as heart valve, and increased food production.
bioethicists People concerned with the ethical questions that arise in the relationships among biology, biotechnology, medicine, politics, law, and philosophy.
bioinformatics An interdisciplinary field which helps solve biological problems using com- puters; may also be called computational biology.
bioremediation The use of microorganisms to clean up contaminated sites, such as an oil spill.
biotechnology Technology based on biology; it involves the use of organisms or biological processes and can be especially used in agriculture, food science, and medicine.
conflict of interest A situation in which a researcher has professional or personal interests that are at odds with each other.
euthanasia The choice by a terminally ill person to have medical assistance in dying.
ethics The discipline concerned with what is morally good and bad, right and wrong.
peer review The process of opening a scientist’s research or ideas (in the form of a scientific paper) to examination by others scientist who are experts in the same field.
reproducibility The ability to repeat experiments and get the same results.
research scientist A person that does scientific investigations and makes discoveries.
science magazine A publication with news, opinions and reports about science; written for a non-expert audience.
scientific article A scientific article discussing new research and findings; usually pub- lished in a scientific journal.
scientific consensus The collective judgment, position, and opinion of a community of scientists in a particular field of science, at a particular time.
scientific journal A publication that communicate and document the results of research carried out in universities and various other research institutions.
scientific misconduct The violation of standard codes of scholarly conduct and ethical behavior in professional scientific research.
stem cell research Research involving stem cells, usually harvested from human embryos.
systematic bias A bias that is introduced from a flaw in measurements.
Bias can also be introduced into an investigation by uncalibrated or broken equipment. Consider ways to avoid this type of bias in your investigations.
If you had to explain to a younger student the importance of learning biology, how would you go about it?
Rules for correct behavior in the lab include not eating or drinking, dressing correctly, and no horseplay. These rules are for general safety in the lab, but could they also be considered lab ethics?
Identify the units of measurement that scientists use.
Contrast light microscopes and electron microscopes.
Identify three items that are common to science labs.
Outline the importance of mathematics to scientific research.
Outline what students and researchers can do to stay safe while working in the lab.
Scientists need to know they are talking the same language when it comes to measurements and analysis of data. Therefore a “standard language of measurement” called the SI sys- tem is used in scientific research. Other standard procedures and techniques are carried out so that scientists from around the world can understand what was done to get to a particular conclusion. These involve standard laboratory procedures and equipment, such as microscopes.
The measurements that scientists use are based on the International System of Units (SI), which is a form of the metric system. The term SI is shortened from the French term Le Système international d’unités. It is the world’s most widely used system of units, both in science and business. It is useful to scientists because it is based on multiples of 10. The SI was developed in 1960 from an older metric system and is used in almost every country.
The SI is not static, as the technology of measurement progresses, units are created and definitions are changed through international agreement among many nations. The interna- tional system of units is made up of a seven base units, shown in Table 2.2 ?? lists SI Base Units. From these seven base units several other units are derived.
Table 1.2: | SI Base Units | ||
Name | Symbol | Quantity | |
meter kilogram second ampere kelvin | m kg s A K | length mass time electric current thermal energy (tempera- ture) | |
53 |
Table 1.2: (continued)
Name | Symbol | Quantity |
mole | mol | amount of substance |
candela | cd | luminous intensity |
A prefix may be added to SI units to make a multiple of the original unit. An SI prefix is a name or symbol that is put before a unit of measure (or its symbol) to form a decimal or a multiple of the unit. For example, kilo- is a multiple of a thousand and milli- is a multiple of a thousandth, so there are one thousand millimeters in a meter, and one thousand meters in a kilometer. All prefixes are multiples of 10, as you can see from Table 2.3 ?? lists SI Prefixes. The prefixes are never combined; a millionth of a kilogram is a milligram not a microkilogram.
Table 1.3: SI Prefixes
Name | Symbol | Factor of 10 | |
tera- | T | 1,000,000,000,000 | trillion (thousand |
(1012) | billion) | ||
giga- | G | 1,000,000,000 (109) | billion (thousand |
million) | |||
mega- | M | 1,000,000 (106) | million |
kilo- | k | 1000 (103) | thousand |
hecto- | h | 100 (102) | hundred |
deca- | da | 10 (101) | ten |
deci- | d | 1 (10-1) | tenth |
centi- | c | 0.1 (10-2) | hundredth |
milli- | m | 0.01 (10-3) | thousandth |
micro- | µ | 0.00001 (10-6) | millionth |
nano- | n | 0.00000001 (10-9) | billionth |
pico- | p | 0.00000000001 | trillionth |
(10-12) |
A laboratory is a place that has controlled conditions in which scientific research, experi- ments, and measurement may be carried out. Scientific laboratories can be found in schools and universities, in industry, in government facilities, and even aboard ships and spacecraft, such as the one shown in Figure 1.27.
Because of the different areas of science, there are many different types of science labs that www.ck12.org 54

each include different scientific equipment. For example, a physics lab might contain a particle accelerator, in which the particles that make up atoms are studied. A chemistry or biology lab most likely contains a fume hood where substances with poisonous fumes can be worked. A particle accelerator and a fume hood are both shown in Figure 1.28. Despite the great differences among labs, some features are common in them.
Most labs have workbenches or countertops at which the scientist may sit or stand to do work comfortably. This is important because scientists can spend all day working in the lab. A scientist usually records an experiment’s progress in a lab notebook, but modern labs almost always contain a computer for data collection and analysis. In many labs computers are also used for lab simulations (modeling or imitating an experiment or a natural process), and for presenting results in the form of graphs or tables.

Lab techniques include the procedures used in science to carry out an experiment. Lab tech- niques follow scientific methods, and while some of them involve the use of simple laboratory equipment such as glassware (shown on the shelves in Figure 1.27), others use more complex
and expensive equipment such as electrical and computerized machines such as the particle accelerator shown in Figure 1.28, or use expensive supplies.
Equipment commonly found in a biology labs include microscopes, weighing scales or bal- ances, water baths, glassware (such as test tubes, flasks, and beakers), Bunsen burners, tongs, pipettes shown in Figure 1.29, chemical reagents, lab coats, goggles, and biohazard waste containers.

µL, which is smaller than a drop of water. (16)
Microscopes are instruments used to view objects that are too small to be seen by the naked eye. Optical microscopes, such as the one shown in Figure 1.30, use visible light and lenses to magnify objects. They are the simplest and most widely used type of microscopes. Compound microscopes are optical microscopes which have a series of lenses, and have uses in many fields of science, particularly biology and geology. The scientist in Figure 1.31 is looking through a compound light microscope that is fitted with a digital camera.
Resolution is a measure of the clarity of an image; it is the minimum distance two points can be separated and still be distinguished as two separate points. Because light beams have a physical size, which is described in wavelengths, it is difficult to see an object that is about the same size or smaller than the wavelength of light. Objects smaller than about 0.2 micrometers appear fuzzy, and objects below that size cannot be seen.
Magnification involves enlarging the image of an object so that it appears much bigger than its actual size. Magnification also refers to the number of times an object is magnified. For example, a lens that magnifies 100X, magnifies an object 100 times larger than its actual size. Light microscopes have three objective lenses that have different magnifications, as
www.ck12.org 56

shown in Figure 1.32. The ocular lens has a magnification of 10X, so a 100X objective lens and the ocular lens together will magnify an object by 1000X.

Figure 1.32: Objective lenses of a light microscope. (48)
Visible light has wavelengths of 400 to 700 nanometers, which is larger than many objects of interest such as the insides of cells. Scientists use different types of microscopes in order to get better resolution and magnification of objects that are smaller than the wavelength of visible light. Objects that are to be viewed under an electron microscope may need to be specially prepared to make them suitable for magnification.
www.ck12.org 58
Electron microscopes use electrons instead of photons (light), because electrons have a much shorter wavelength than photons and thus allow a researcher to see things at very high magnification, far higher than an optical microscope can possibly magnify.
There are two general types of electron microscopes: the Transmission Electron Microscope that shoots electrons through the sample and measures how the electron beam changes because it is scattered in the sample, and the Scanning Electron Microscope that scans an electron beam over the surface of an object and measures how many electrons are scattered back.
Transmission electron microscopy (TEM) is an imaging method in which a beam of electrons is passed through a specimen. An image is formed on photographic film or a fluorescent screen by the electrons that scatter when passing through the object. TEM images show the inside of the object.
The scanning electron microscope (SEM) is a type of electron microscope capable of producing high-resolution images of a sample surface. Due to the manner in which the image is created, SEM images have a characteristic three-dimensional appearance and are useful for judging the surface structure of the sample. Sometimes objects need to be specially prepared to make them better suited for imaging under the scanning electron microscope, as shown with the insect in Figure 1.33.
Electron microscopes work under low pressures and usually in a vacuum chamber to avoid scattering the electrons in the gas. This makes the microscopes considerably larger and more expensive than optical microscopes. The different types of images from the two electron microscopes are shown in Figure 1.34.
In the microbiology lab, aseptic technique refers to the procedures that are carried out under sterile conditions. Scientists who study microbes are called microbiologists. Micro- biologists must carry out their lab work using the aseptic technique to prevent microbial contamination of themselves, contamination of the environment they are working in, in- cluding work surfaces or equipment, and contamination of the sample they are working on. Bacteria live on just about every surface on Earth, so if a scientist wants to grow a particular type of bacterium in the lab, he or she needs to be able to sterilize their equipment to prevent contamination by other bacteria or microorganisms. The aseptic technique is also used in medicine, where it is important to keep the human body free of contamination.
Aseptic technique is used whenever bacteria or other microbes are transferred between nu- trient media or in the preparation of the nutrient media. Some equipment that is used in the aseptic technique include a Bunsen burner, an autoclave (Figure 1.35), hand and surface sanitizers, neoprene gloves, and a fume hood.

Figure 1.34: SEM and TEM images of the algae Chlamydomonas. The SEM image, shown at the right, is a three-dimensional image of the surface of the organism, whereas the TEM image is a two-dimensional image of the interior of the organism. (32)
www.ck12.org 60
Students of microbiology are taught the principles of aseptic technique by hands-on labora- tory practice. Practice is essential in learning how to handle the lab tools without contami- nating them.

Scientific models are representations of reality. To describe particular parts of a phenomenon, or the interactions among a set of phenomena, it is sometimes helpful to develop a model of the phenomenon. For instance, a scale model of a house or of a solar system is clearly not an actual house or an actual solar system; the parts of an actual house or an actual solar system represented by a scale model are, only in limited ways, representative of the actual objects.
Scientific modeling is the process of making abstract models of natural phenomena. An abstract model is a theoretical construct that represents something. Models are developed to allow reasoning within a simplified framework that is similar to the phenomena being investigated. The simplified model may assume certain things that are known to be incom- plete in some details. Such assumptions can be useful in that they simplify the model, while at the same time, allowing the development of acceptably accurate solutions. These models play an important role in developing scientific theories.
A simulation is a model that runs over time. A simulation brings a model to life and

shows how a particular object or phenomenon will behave. It is useful for testing, analysis or training where real-world systems or concepts can be represented by a model. For the scientist, a model also provides a way for calculations to be expanded to explore what might happen in different situations. This method often takes the form of models that can be programmed into computers. The scientist controls the basic assumptions about the variables in the model, and the computer runs the simulation, eventually coming to a complicated answer.
Examples of models include:
Computer models
Weather forecast models
Molecular models
Climate models
Ecosystem models
Geologic models
One of the main aims of scientific modeling is to allow researchers to quantify their obser- vations about the world. In this way, researchers hope to see new things that may have escaped the notice of other researchers. There are many techniques that model builders use which allow us to discover things about a phenomenon that may not be obvious to everyone.
www.ck12.org 62
The National Weather Service Enhanced Radar Images web site (http://radar. weather.gov/) is an excellent example of a simulation. The site exhibits current weather forecasts across the United States.
A person who builds a model must be able to recognize whether a model reflects reality. They must also be able to identify and work with differences between actual data and theory.
A model is evaluated mostly by how it reflects past observations of the phenomenon. Any model that is not consistent with reproducible observations must be modified or rejected. However, a fit to observed data alone is not enough for a model to be accepted as valid. Other factors important in evaluating a model include:
Its ability to explain past observations
Its ability to predict future observations
Its ability to control events
The cost of its use, especially when used with other models
Ease of use and how it looks
Some examples of the different types of models that are used by science are shown in Figures
1.37 and 1.38.
Theories are constructed in order to explain, predict and understand phenomena. This could include the movement of planets, weather patterns, or the behavior of animals, for example. In many instances we are constructing models of reality. A theory makes generalizations about observations and is made up of a related set of ideas and models. The important difference between theories and models is that the first is explanatory as well as descriptive, while the second is only descriptive and predictive in a much more limited sense.
In some laboratories, conditions are no more dangerous than in any other room. In many labs, though, additional hazards are present. Laboratory hazards are as varied as the subjects of study in laboratories, and might include poisons, infectious agents, flammable, explosive, or radioactive materials, moving machinery, extreme temperatures, or high voltage. The hazard symbols for corrosive, explosive, and flammable substances are shown in Figure 1.39. In laboratories where conditions might be dangerous, safety precautions are important. Lab

Figure 1.38: Biosphere 2 is an example of a very large three-dimensional model which biol- ogists built to attempt to recreate a self-sustaining biome. To learn more about biomes and ecosystems, go to the Biomes, Ecosystems and Communities chapter. (5)
www.ck12.org 64

Figure 1.39: The hazard symbols for corrosive, explosive, and flammable substances. (40)
safety rules minimize a person’s risk of getting hurt, and safety equipment is used to protect the lab user from injury or to help in responding to an emergency.
Some safety equipment that you might find in a biology lab includes:
Sharps Container A container that is filled with used medical needles and other sharp instruments such as blades, shown in Figure 1.40. Needles or other sharp items that have been used are dropped into the container without touching the outside of the container. Objects should never be pushed or forced into the container, as damage to the container or injuries may result.
Laminar Flow Cabinet A carefully enclosed bench designed to prevent contamination of biological samples. Air is drawn through a fine filter and blown in a very smooth, laminar (streamlined) flow towards the user. The cabinet is usually made of stainless steel with no gaps or joints where microorganisms might collect.
Gloves Due to possible allergic reactions to latex, latex gloves are not recommended for lab use. Instead, vinyl or nitrile gloves, shown in Figure 1.41, are often used. Gloves protect the wearers hands and skin from getting contaminated by microorganisms or stained or irritated by chemicals.
Lab Coat A knee-length overcoat that is usually worn while working in the lab. The coat www.ck12.org 66
helps to protect the researcher’s clothes from splashes or contamination. The garment is made from white cotton or linen to allow it to be washed at high temperature and make it easy to see if it is clean.
Safety precautions are in place to help prevent accidents. Always wear personal protective equipment such as goggles and gloves when recommended to do so by your teacher.
Tell your teacher immediately if an accident happens.
The production of aerosols due to poor technique such as squirting the last drop out of pipettes, and the spread of contamination due to spills is completely avoidable and especially important if you are handling infectious material or chemicals.
Wear enclosed toe shoes, instead of sandals or flip flops, or thongs. Your feet and toes could easily get hurt or broken or if you dropped something. (Figure 1.42)
Do not wear loose, floppy clothes in the lab; they can get caught in or knock over equipment, causing an accident.
If you have long hair, tie it up for the same reasons listed above.
Do not eat or drink in the lab.
Do not use cell phones in the lab, even if you are only sending a text message. You can easily contaminate your phone with whatever you have been working with. Consider where your hands have been, and where your face will be the next time you talk on the phone.
Sweep up broken glass immediately and dispose in a designated area or container, or notify your teacher.
Always listen carefully to your teacher’s instructions.

In the case of an accident, it is important to begin by telling your teacher and to know where to find safety equipment.
Some common safety equipment in a school lab:
Fire Extinguishers
Fire Blanket
Eye-Wash Fountain (Figure 1.43)
First-Aid Kit
Figure 1.43: Symbol for the eyewash fountain. (51)
Through the first three lessons, we have discussed what science is and how science is done. Now we need to turn our attention to Biology. Biology is the study of life. As the ‘study of life,’ a knowledge of biology is an extremely important aspect of your education. Biology includes the identification and analysis of characteristics common to all living organisms. What is known about biology is discovered or identified through the same processes as all other sciences, including the scientific method and peer review process.
The measurements that scientists use are based on the International System of Units (SI), which is form of the metric system. Based on multiples of ten, It is the world’s most widely used system of units, both in science and business.
One important use for mathematics in science is the role it plays in expressing scientific models. Statistics allow scientists to assess the reliability and range of differences in experimental results.
www.ck12.org 68
Light microscopes use visible light and lenses to magnify objects. They are the simplest and most widely used type of microscopes. Electron microscopes use electrons instead of photons (light), because electrons have a much shorter wavelength than photons and therefore allow a researcher to see things at very high magnification, that greatly exceeds what an optical microscope can possibly magnify. Electron microscopes are larger and more expensive than light microscopes.
Equipment commonly found in a biology labs include microscopes, weighing scales or balances, water baths, glassware (such as test tubes, flasks, and beakers), Bunsen burners, tongs, pipettes, chemical reagents, lab coats, goggles, and biohazard waste containers.
Always wear personal protective equipment such as goggles and gloves, wear enclosed shoes, and do not eat or drink in the lab.
Which one of the following units of measurement would be the most appropriate in determining the mass of a banana? Kilograms, micrograms, or grams.
Identify the type of microscope that is most common in laboratories.
Contrast microscope magnification and resolution.
If an objective lens magnifies an object by 45×, and the optical lens magnifies by 10×. By how much will the object be magnified to the viewer?
Which object is larger? An object with a diameter of 1500 micrometers (µm) or an object with a diameter of 15 millimeters (mm)?
Why is it important that scientists use common units of measurement?
Name three pieces of safety equipment that you should wear while carrying out an investigation in the lab.
What should you first do if an accident happens in the lab?
If you saw this hazard sign on a chemical container, what do you think it might mean?

How are computer models similar to the real world, and how do they differ?
http://en.wikibooks.org/wiki/Nanotechnology/Electron_microscopy
aseptic technique Laboratory procedures that are carried out under sterile conditions.
compound microscope An optical microscopes that has a series of lenses, and have uses in many fields of science, particularly biology and geology.
electron microscope A microscope that uses electrons instead of light; allow a researcher to see things at very high magnification, far higher than an optical microscope can possibly magnify.
International System of Units (SI) The measurements that scientists use; a form of the metric system.
lab coat A knee-length overcoat that is usually worn while working in the lab; helps to protect the researcher’s clothes from splashes or contamination.
laboratory A place that has controlled conditions in which scientific research, experiments, and measurement may be carried out.
lab techniques The procedures used in science to carry out an experiment.
magnification Enlarging an image of an object so that it appears much bigger than its actual size; also refers to the number of times an object is magnified.
microscopes Instruments used to view objects that are too small to be seen by the naked eye.
model A physical, mathematical, or logical representation of a system, phenomenon, or process; allow scientists to investigate a phenomenon in a controlled way.
optical microscope A microscope that uses visible light and lenses to magnify objects.
resolution A measure of the clarity of an image; it is the minimum distance two points can be separated and still be distinguished as two separate points.
www.ck12.org 70
scanning electron microscope (SEM) Electron microscope that scans an electron beam over the surface of an object and measures how many electrons are scattered back.
scientific modeling The process of making abstract models of natural phenomena.
simulation A model that runs over time; brings a model to life and shows how a particular object or phenomenon will behave.
stereo microscope A light microscope with two ocular lenses.
transmission electron microscope (TEM) Electron microscope that shoots electrons through the sample and measures how the electron beam changes because it is scattered in the sample.
Consider how much more difficult it would be to carry out investigations without the use of computers, and the types of models that have developed due to the development of computers.
Consider reasons why eating and drinking are not allowed in the lab.
What additional ethical considerations would there be if you were working with living organisms in the lab, such as mice, rats, or other mammals?
List some of the different areas of study in biology.
Identify the seven characteristics of living things.
Identify the four unifying principles of modern biology.
List two different types of interactions that organisms can have with each other.
Outline the formation of modern evolutionary theory.
Biology examines the structure, function, growth, origin, evolution, and distribution of living things. It classifies and describes organisms, their functions, how species come into existence, and the interactions they have with each other and with the natural environment. Four
unifying principles form the foundation of modern biology: cell theory, evolution, genetics and homeostasis.
Most biological sciences are specialized areas of study. Biology includes biochemistry, cell biology, microbiology, immunology, genetics, physiology, zoology, ecology, evolutionary bi- ology, and botany. Biochemistry is the study of the chemicals that make up life. Cell biology is the study of life at the level of the cell. Microbiology is the study of microscopic organisms. Immunology is the study of an organism’s resistance to disease. Genetics is the study of how organisms pass traits to their offspring. The study of how the human body works is called physiology. Zoology is the study of animals. The study of how organisms interact with their environment and each other is called ecology. Evolutionary biology is the study of how populations and species change over time. Botany is the study of plants. The four unifying principles are important foundations for each and every field of biology. Applied fields of biology such as medicine and genetic research involve many specialized areas of study.
Not all scientists agree exactly about what makes up life. Many characteristics describe most living things. However, with most of the characteristics listed below we can think of one or more examples that would seem to break the rule, with something non-living being classified as living or something living classified as non-living.
There is not just one distinguishing feature that separates a living thing from a non-living thing. A cat moves but so does a car. A tree grows bigger, but so does a cloud. A cell has structure, but so does a crystal. Biologists define life by listing characteristics that living things share. Something that has all of the characteristics of life is considered to be alive. The duck decoy in Figure 1.44 may look like a duck, act like a duck in that it floats about, but it is not alive. The decoy cannot reproduce offspring, respond to its environment, or breathe.

An individual living creature is called an organism. There are many characteristics that www.ck12.org 72
living organisms share. They all:
respond to their environment
grow and change
reproduce and have offspring
have a complex chemistry
maintain homeostasis
are built of structures called cells
pass their traits onto their offspring
If you step on a rock, it will just lie there, but if you step on a turtle, it may move or even snap at you. Living things know what is going on around them, and respond to changes in the environment. An adaptation refers to the process of becoming adjusted to an environment. Adaptations may include structural, physiological, or behavioral traits that improve an organism’s likelihood of survival, and thus, reproduction.
A seed may look like a pebble, but under the right conditions it will sprout and form a seedling that will grow into a larger plant. The pebble of course will not grow.
Living things make more organisms like themselves. Whether the organism is a rabbit, or a tree, or a bacterium, life will create more life.
A flower has a complicated and beautiful structure. So does a crystal. But if you look closely at the crystal, you see no change. The flower, on the other hand, is transporting water through its petals, producing pigment molecules, breaking down sugar for energy, and undergoing a large number of other chemical reactions that are needed for living organisms to stay alive. We call the sum of the chemical reactions in a cell its metabolism.
A human body has a temperature of 37° Celsius, (about 98.6° Fahrenheit). If you step outside on a cold morning, the temperature might be below freezing. Nevertheless, you do
not become an ice cube. Your shiver and move your arms and legs about to stay warm. Eating food also gives your body the energy to keep warm. Living organisms keep their internal environments within a certain range (they maintain a stable internal condition), despite changes in their external environment. This process is called homeostasis.
If you look closely at any organism you can see that it is made of structures called cells. Organisms that are very different such as ferns, and fish, and elephants all look very similar at the cellular level. All living organisms are made of one or more cells. Organisms are organized in the microscopic level from atoms up to cells. The matter is structured in an ordered way. Atoms are arranged into molecules, then into macromolecules, which make up organelles, which work together to form cells. Beyond this, cells are organized in higher levels to form entire multicellular organisms, as shown in Figure 1.45. Cells together form tissues, which make up organs, which are part of organ systems, which work together to form an entire organism. Of course, beyond this, organisms form populations which make up parts of an ecosystem. All of Earth’s ecosystems together form the diverse environment that is Earth.
There are four unifying principles of biology that are important for types of biology studies. These are:
www.ck12.org 74
The cell is the basic unit of life. The Cell Theory states that all living things are made of one or more cells, or the secretions of those cells, such as the organisms shown in Figure 1.46. For example, shell and bone are built by cells from substances that they secrete into their surroundings. Cells come from cells that already exist, that is, they do not suddenly appear from nowhere. In organisms that are made of many cells (called multicellular organisms), every cell in the organism’s body derives from the single cell that results from a fertilized egg. You will learn more about cells and the Cell Theory in the Cell Structure and Function chapter.
A living organism’s traits are encoded in their DNA, the large molecule, or macromolecule, that holds the instructions needed to build cells and organisms. DNA makes up the genes of an organism. Traits are passed on from one generation to the next by way of these genes. Information for how the organism appears and how its cells work come from the organism’s genes. Although the appearance and cell function of the organism may change due to the organism’s environment, the environment does not change its genes. The only way that genes can change in response to a particular environment is through the process of evolution in populations of organisms. You will learn more about DNA and genes in the Molecular Genetics chapter.
Homeostasis is the ability of an organism to control its body functions in order to uphold a stable internal environment even when its external environment changes. All living organ- isms perform homeostasis. For example, cells maintain a stable internal acidity (pH); and warm-blooded animals maintain a constant body temperature. You will learn more about homeostasis in The Human Body chapter.
Homeostasis is a term that is also used when talking about the environment. For exam- ple, the atmospheric concentration of carbon dioxide on Earth has been regulated by the concentration of plant life on Earth because plants remove more carbon dioxide from the atmosphere during the daylight hours than they emit to the atmosphere at night.
Evolution by natural selection, is the theory that maintains that a population’s inherited traits change over time, and that all known organisms have a common origin. Evolutionary theory can explain how specialized features, such as the geckos sticky foot pads shown in Figure 1.47, develop in different species. You will learn more about evolution in the Evolutionary Theory and Evolution in Populations chapters.

www.ck12.org 76
Biological interactions are the interactions between different organisms in an environment. In the natural world no organism is cut off from its surroundings. Organisms are a part of their environment which is rich in living and non-living elements that interact with each other in some way. The interactions of an organism with its environment are vital to its survival, and the functioning of the ecosystem as a whole.
These relationships can be categorized into many different classes. The interactions between two species do not necessarily need to be through direct contact. Due to the connected nature of ecosystems, species may affect each other through such relationships involving shared resources or common enemies.
The term symbiosis comes from a Greek word that means “living together.” Symbiosis can be used to describe various types of close relationships between organisms of different species, such as mutualism and commensalism, which are relationships in which neither organism is harmed. Sometimes the term symbiosis is used only for cases where both organisms benefit, sometimes it is used more generally to describe all kinds of close relationships, even when one organism is killed by another, as shown in Figure 1.48. Symbiosis can also be used to describe relationships where one organism lives on or in another, called parasitism, or when one organism kills and eats another organism, called predation.
Competition is as an interaction between organisms or species, for the same resources such as water, food, or hunting grounds in an environment, shown in Figure 1.49. Eventually, the species that is less able to compete for resources will either adapt or die out. According to evolutionary theory, competition for resources plays an important role in natural selection.
Animals that eat decomposing organic material also have an important interaction with the environment. They help to decompose dead matter and assist with the recycling of nutrients. By burying and eating dung, dung beetles, such as the one shown in Figure 1.50, improve nutrient cycling and soil structure. They make the dead organic matter available to bacteria that break it down even further.
In studying how organisms interact with each other, biologists often find it helpful to classify the organisms and interactions into levels of organization. Similar to the way an organism itself has different levels of organization, the ways in which organisms interact with their environment and each other can also be divided in to levels of organization. For example:
The biosphere includes all living things within all of their environments. It includes every

Figure 1.48: There are many different types of symbiotic interactions between organisms. Clockwise from top left: Escherichia coli bacteria live inside your intestines in a mutualistic relationship; the bacteria produce Vitamin K for you, and they get their food from what you eat. Lions are predators that feed on other organisms such as this Cape buffalo. Similar to the E. coli, this bee has a mutualistic relationship with the flower, the bee feeds from the flower, and the flower gets pollinated by the bee. Clownfish that live among the tentacles of sea anemones protect the anemone from anemone-eating fish, and in turn the stinging tentacles of the anemone protect the clownfish from its predators (a special mucus on the clownfish protects it from the stinging tentacles). (23)

www.ck12.org 78

place that life occurs, from the upper reaches of the atmosphere to the top few meters of soil, to the bottoms of the oceans. An ecosystem is made up of the relationships among smaller groups of organisms with each other, and their environment. Scientists often speak of the interrelatedness of living things, because, according to evolutionary theory, organisms adapt to their environment, and they must also adapt to other organisms in that environment.
A community is made up of the relationships between groups of different species. For example, the desert communities consist of rabbits, coyotes, snakes, birds, mice and such plants as sahuaro cactus, ocotillo, and creosote bush. Community structure can be disturbed by such dynamics as fire, human activity, and over-population.
It is thus possible to study biology at many levels, from collections of organisms or commu- nities, to the inner workings of a cell (organelle). To learn more about the interactions of organisms, you will read the Biomes, Ecosystems and Communities and Populations chap- ters.
Evolutionary theory and the cell theory give us the basis for how and why, living things relate to each other. The diversity of life found on Earth today is the result of 4 billion years of evolution. Some of this diversity is shown in Figure 1.51. The origin of life is not completely understood by science, though limited evidence suggests that life may already have been well-established a few 100 million years after Earth formed. Until approximately 600 million years ago, all life was made up of single-celled organisms.
The level of biodiversity found in the fossil record suggests that the last few million years include the period of greatest biodiversity in the Earth’s history. However, not all scientists support this view, since there is a lot of uncertainty as to how strongly the fossil record is biased by the greater availability and preservation of more recent fossil-containing rock layers. Some researchers argue that modern biodiversity is not much different from biodiversity 300 million years ago. Estimates of the present global species diversity vary from 2 million to 100 million species, with a best estimate of somewhere near 10 million species. All living organisms are classified into one of the six kingdoms: Archaebacteria (Archaea), Eubacteria (Bacteria), Protista (Protists), Fungi, Plantae (Plants), and Animalia (Animals).
New species are regularly discovered and many, though already discovered, are not yet clas- sified. One estimate states that about 40 percent of freshwater fish from South America are not yet classified. Every year, scientists discover the existence of many hundreds more archaea and bacteria than were previously known about. Just a few of the many members of the animal kingdom are shown in Figure 1.51. The animal kingdom is just a tiny portion of the total diversity of life. To learn more about the diversity of living creatures, you will read the Classification; Prokaryotes and Viruses; Protists; Fungi; Evolution and Classification of Plants; and Introduction to Animals and Invertebrates chapters.
Evolution is the process by which populations of organisms change over time. These organ- isms acquire and pass on new traits from generation to the next generation. Its occurrence over large stretches of time explains the origin of new species and the great diversity of the biological world. Extant species are related to each other through common descent, and products of evolution over billions of years. Analysis of the DNA of different organisms indicate there is a similarity in the DNA genetic codes that help make proteins and other molecules in very different organisms. These genetic codes are used by all known forms of life on Earth, and are very similar. The theory of evolution suggests that the genetic code was established very early in the history of life and some studies suggest it was established soon after the formation of Earth. The timeline of the evolution of life, shown in Figure 1.52, outlines the major events in the development of life.
How do scientists know Earth is so old? The answer is in the rocks. Contained in rocks that were once molten, shown in Figure 1.53, are chemical elements that act like an atomic clock. The atoms of different forms of elements (called isotopes) break down at different rates over time. Parent isotopes within these rocks decay at a predictable rate to form daughter isotopes. By determining the relative amounts of parent and daughter isotopes, the age of these rocks can be calculated—forming the so-called atomic clock.
Thus, the results of studies of rock layers (stratigraphy), and of fossils (paleontology), along with the ages of certain rocks as measured by atomic clocks (geochronology), indicate that the Earth is over 4.5 billion years old, with the oldest known rocks being 3.96 billion years
www.ck12.org 80

www.ck12.org 82
old. To learn more about the history of life on Earth, you will read the History of Life chapter.

Figure 1.53: Molten rock, called lava, is expelled by a volcano during an eruption. The lava will eventually cool to become solid rock. When first expelled from a volcanic vent, it is a liquid at temperatures from 700 °C to 1,200 °C (1,300 °F to 2,200 °F). Not all types of rocks come from cooled lava, but many do. Additional images/videos of volcanic eruptions can be seen at Hawaii Volcanic Eruption with Lightning and USGS Kilauea Vol- cano (http://www.youtube.com/watch?v=y3aqFCT87_E and http://hvo.wr.usgs.gov/ gallery/kilauea/volcanomovies/). (36)
The theory of evolution by natural selection was proposed at about the same time by both Charles Darwin and Alfred Russel Wallace, shown in Figure 1.54, and was set out in detail in Darwin’s 1859 book On the Origin of Species. Natural selection is a process that causes heritable traits that are helpful for survival and reproduction to become more common, and harmful traits, or traits that are not helpful or advantageous for survival to become more rare in a population of organisms. This occurs because organisms with advantageous traits are more ”fit” to survive in a particular environment and have ”adapted” to the conditions of that environment. These individuals will have greater reproductive success than organisms less fit for survival in the environment. This will lead to an increase in the number of organisms with the advantageous trait(s) over time. Over many generations, adaptations occur through a combination of successive, small, random changes in traits, and natural selection of those variants best-suited for their environment. Natural selection is one of the cornerstones of modern biology.
The theory of evolution encountered initial resistance from religious authorities who believed humans were divinely set apart from the animal kingdom. There was considerable concern about Darwin’s proposal of an entirely scientific explanation for the origin of humans. Many people found such an explanation to be in direct conflict with their religious beliefs. A caricature of Darwin as a monkey, shown in Figure 1.55, reflects the controversy that arose

over evolutionary theory. In the 1930s, Darwinian natural selection was combined with Mendelian inheritance to form the basis of modern evolutionary theory.

The identification of DNA as the genetic material by Oswald Avery and colleagues in the 1940s, as well as the publication of the structure of DNA by James Watson and Francis Crick in 1953, demonstrated the physical basis for inheritance. Since then, genetics and molecular biology have become core aspects of evolutionary biology.
Currently the study of evolutionary biology involves scientists from fields as diverse as bio- chemistry, ecology, genetics and physiology, and evolutionary concepts are used in even more
www.ck12.org 84
distant disciplines such as psychology, medicine, philosophy and computer science.
The following list includes some common misconceptions about evolution.
The term evolution describes the changes that occur in populations of living organisms over time. Describing these changes does not address the origin of life. The two are commonly and mistakenly confused. Biological evolution likewise says nothing about cosmology, the Big Bang, or where the universe, galaxy, solar system, or Earth came from.
Humans did not evolve from chimpanzees or any other modern ape; instead they share a common ancestor that existed around 7 million years ago.
The process of evolution is not necessarily slow. Millions of years are not required to see evolution in action. Indeed, it has been observed multiple times under both controlled laboratory conditions and in nature.
Evolution is not a progression from ”lower” to ”higher” forms of life, and it does not increase in complexity. For example, bacteria have simpler structures and a smaller amount of genetic material than humans do. This does not mean however, that bacteria are “less evolved” than humans are. Bacteria have evolved over many millions of years and are well adapted to their own environments.
Since Darwin’s time, scientists have gathered a more complete fossil record, including mi- croorganisms and chemical fossils. These fossils have supported and added more information to Darwin’s theories. However, the age of the Earth is now held to be much older than Dar- win thought. Researchers have also uncovered some of the preliminary mysteries of the mechanism of heredity as carried out through genetics and DNA, which were areas unknown to Darwin. Another growing subject is the study of comparative anatomy, which looks at how different organisms have similar body structures. Molecular biology studies of slowly changing genes reveal an evolutionary history that is consistent with fossil and anatomical records.
Biochemistry is the study of the chemicals that make up life. Cell biology is the study of life at the level of the cell. Microbiology is the study of microscopic organisms. Genetics is the study of how organisms pass traits to their offspring. The study of how the human body works is called physiology. Zoology is the study of animals. The study of how organisms interact with their environment and each other is called
ecology. Evolutionary biology is the study of how populations and species of animals change over time. Botany is the study of plants.
The seven characteristics of life include: responsiveness to the environment; growth and change; ability to reproduce; have a metabolism and breathe; maintain homeostasis; being made of cells; passing traits onto offspring.
Four unifying principles form the foundation of modern biology: cell theory, evolution, genetics and homeostasis. These four principles are important to each and every field of biology.
Symbiosis can be used to describe various types of close relationships between organ- isms of different species, such as mutualism and commensalism, which are relationships in which neither organism is harmed. Sometimes the term symbiosis is used only for cases where both organisms benefit, but sometimes it is used more generally to de- scribe all kinds of close relationships, even when one organism is killed by another. Symbiosis can also be used to describe relationships where one organism lives on or in another, called parasitism, or when one organism kills and eats another organism, called predation. Competition is as an interaction between organisms or species for the same resources in an environment.
Analysis of the DNA of different organisms indicate that there is a similarity in the DNA genetic codes that help make proteins and other molecules in very different organisms. These genetic codes are used by all known forms of life on Earth, and are very similar. The theory of evolution suggests that the genetic code was established very early in the history of life and some studies suggest it was established soon after the formation of Earth.
Identify three of the seven characteristics of living things.
Identify the four unifying principles of modern biology.
List two different types of interactions that organisms can have with each other.
Outline the formation of modern evolutionary theory.
Give an example of how you are interdependent from another organism.
You find an object that looks like a dead, brown leaf, but it also looks like it might have eyes and legs–features that leaves do not usually have. How would you go about determining if this object was a living creature.
What is the basic unit of life?
What is homeostasis?
How have more recent scientific findings fit with evolutionary theory since Darwin’s time?
Large animals are more evolved than single-celled organisms such as bacteria. Do you agree with this statement?
www.ck12.org 86
http://en.wikibooks.org/wiki/Biology%2C_Answering_the_Big_Questions_of_Life/ Introduction
http://www.ucmp.berkeley.edu/help/timeform.html
adaptation Refers to the process of becoming adjusted to an environment; may include structural, physiological, or behavioral traits that improve an organism’s likelihood of survival and reproduction.
biochemistry The study of the chemicals that make up life.
biological interactions The interactions between different organisms in an environment.
biology The study of life.
biosphere Every place that life occurs, from the upper reaches of the atmosphere to the top few meters of soil, to the bottoms of the oceans.
botany The study of plants.
cell The smallest unit of structure and function of living organisms.
cell biology The study of life at the level of the cell.
community Composed of the relationships between groups of different species.
competition An interaction between organisms or species, for the same resources such as water, food, or hunting grounds in an environment.
ecology The study of how organisms interact with their environment and each other.
ecosystem Made up of the relationships among smaller groups of organisms with each other, and their environment.
evolution The process by which populations of organisms change over time by acquiring and passing on new traits from generation to generation.
evolutionary biology The study of how populations and species change over time. genetics The study of how organisms pass traits to their offspring (heredity). homeostasis The ability to keep an internal environment within a certain range, despite
changes in the external environment.
immunology The study of an organism’s resistance to disease. metabolism The sum of the chemical reactions in a cell. microbiology The study of microscopic organisms.
natural selection A process that causes heritable traits that are helpful for survival and reproduction to become more common, and harmful traits, or traits that are not helpful or advantageous for survival to become more rare in a population of organisms.
organism An individual living creature.
physiology The study of how the human body works.
symbiosis Various types of close relationships between organisms of different species; comes from a Greek word that means ”living together.”
zoology The study of animals.
All modern scientific disciplines support the theory of evolution. Consider what type of hypothesis could be made that might challenge evolutionary theory. Likewise, consider what type of hypothesis could challenge the cell theory.
As you read through other chapters in this book, it might help to remember that study- ing biology does not just mean learning facts by memory or repetition. By studying biology you are developing a knowledge and understanding of the world around you. And, combined with your study of other subjects such as literature, social studies, art, music, mathematics, and physical sciences, you will develop a fuller, deeper understand- ing of what it is to be a human being who interacts with and lives an interdependent life with other organisms (including other humans!) in your environment.
“Intellectual growth should commence at birth and cease only at death.” - Albert Einstein
www.ck12.org 88
ARS (USDA). < . Public Domain.
http://en.wikipedia.org/wiki/Image:Charles_Darwin.jpg. Public Domain.
http://en.wikipedia.org/wiki/Image:Darwin_ape.jpg. Public Domain.
http://en.wikipedia.org/wiki/Image:Sharps_Container.jpg. GNU-FDL.
Colin Marquardt. http://commons.wikimedia.org/wiki/Image:Biosphere2_Inside_big.jpg. Public Domain.
Celia Guthrie, Reed Business Information Ltd. Cover of New Scientist magazine.. GFDL 1.2.
U.S. Federal Government. http://commons.wikimedia.org/wiki/File: Armstrong,_Lance_(December_2007).jpg. Public Domain.
National Institute of General Medical Sciences (Part of the NIH). . Public Domain. (9) http://dx.doi.org/10.1371/journal.pbio.0040101. CC-BY 2.5.
http://commons.wikimedia.org/wiki/Image:Fume_hood.jpg. (a)CRF (b)Public Domain.
FotoosVanRobin. http://www.flickr.com/photos/fotoosvanrobin/1362263183/. CC-BY-SA.
Peter Halasz. http://en.wikipedia.org/wiki/Image:Golden_insect_01_Pengo.jpg. CC-BY-SA 3.0.
Phauly. http://www.flickr.com/photos/phauly/25605309/. CC-BY-SA.
http://commons.wikimedia.org/wiki/Image:Giraffes_Arusha_Tanzania.jpg. GFDL.
NOAA - Air Resources Laboratory. http://www.arl.noaa.gov/data/forecasts/grads/nam/panel1/plt1.gif. Public Domain.
Newbie. http://commons.wikimedia.org/wiki/File:Pipetten.JPG. Public Domain.
Rafael Brix. http://en.wikipedia.org/wiki/Image:Scarabaeus_laticollis.jpg. GFDL.
JJ,Irish Typepad. http://www.flickr.com/photos/irisheyes/85969063/. (a)GFDL 2.1; BT Young Scientist Exhibition (b) CC-BY-SA 2.5.
Roslin Institute. http://www.roslin.ac.uk/imagelibrary/popups/101.php. Free for non-commercial use..
http://dx.doi.org/10.1371/journal.pbio.0020306 http://commons.wikimedia.org/wiki/Image:Whale-shark-enhanced.jpg. (a)CC-BY (b)CC-BY-SA 2.5.
en:User:Justin. http://en.wikipedia.org/wiki/Image:Animal_diversity_October_2007.jpg. CC-BY-SA.
CarbonNYC. http://www.flickr.com/photos/carbonnyc/1925182563/. CC-BY-SA.
http://commons.wikimedia.org/wiki/Image: Male_Lion_and_Cub_Chitwa_South_Africa_Luca_Galuzzi_2004.jpg http://commons.wikimedia.org/wiki/Image:Common_clownfish.jpg http:
//commons.wikimedia.org/wiki/Image:Bees_Collecting_Pollen_cropped.jpg.
Public Domain,CC-BY-SA 2.5,Public Domain,Public Domain.
Chaquetadepollo. http://www.flickr.com/photos/chaquetadepollo/182408691. CC-BY-SA.
USGS. http://en.wikipedia.org/wiki/Image:Geologica_time_USGS.png. Public Domain.
Tjwood. http://en.wikipedia.org/wiki/Image:Disposable_nitrile_glove.jpg. Public Domain.
Malene Thyssen. http://en.wikibooks.org/wiki/Image:Siberian_Tiger_by_Malene_Th.jpg. GFDL.
http://commons.wikimedia.org/wiki/Image:Anousheh_Ansari_in_the_ISS.jpg. Public Domain.
Urocyon, Mwtoews. http://en.wikipedia.org/wiki/Image:Histogram_example.svg. Public Domain, CC-BY-SA.
www.ck12.org 90
http://commons.wikimedia.org/wiki/Image:Chloroplast-new.jpg http://commons.wikimedia.org/wiki/Image:Kasvisolu.png http://commons.wikimedia.org/wiki/Image:Tomato_leaf_x-section_2.jpg http://commons.wikimedia.org/wiki/Image:Leaf_1_web.jpg http://commons.wikimedia.org/wiki/Image:Linde_von_linn.jpg. (a)Public Domain (b)Public Domain (c)GFDL (d)Public Domain (e)Public Domain
(f)CC-BY-SA.
M. Imran. http://en.wikipedia.org/wiki/Image:Research_Journals.jpg. Public Domain.
http://remf.dartmouth.edu/images/algaeTEM/source/1.html. (a)Public Domain (b)Public Domain.
http://commons.wikimedia.org/wiki/Image:Iss013e56052.jpg. (a)CC-BY 1.0 GFDL 1.2 (b)Public Domain.
http://www.flickr.com/photos/vertigogen/1852130723. GFDL 1.2,CC-BY-SA.
Heinz Seehagel,USAID. http://en.wikipedia.org/wiki/Image:BN-forest.jpg. Public Domain,Public Domain.
USGS. http://en.wikipedia.org/wiki/Image:Pahoeoe_fountain_original.jpg. Public Domain.
ARS/USDA. http://commons.wikimedia.org/wiki/Image:Manusingmicroscope.jpg. Public Domain.
Christine Hush, National Science Foundation (NSF). http://photolibrary.usap.gov/Portscripts/PortWeb.dll?query&field1= Filename&op1=matches&value=GENTOOSLMG.JPG&catalog= Antarctica&template=USAPgovMidThumbs. Public Domain.
Florian. http://www.flickr.com/photos/fboyd/661365803/. CC-BY-SA.
http://commons.wikimedia.org/wiki/Image:Hazard_E.svg http://commons.wikimedia.org/wiki/Image:Hazard_F.svg. (c)Public Domain.
GcG,Rozzychan. http://en.wikibooks.org/wiki/Image: Phase_contrast_microscope_labelled1.jpg. Public Domain.
Fruggo. http://commons.wikimedia.org/wiki/Image:Schapen_33.jpg. CC-BY 1.0.
Oren Jack Turner, Princeton, N.J.. http://commons.wikimedia.org/wiki/Image:Albert_Einstein_1947a.jpg. Public Domain.
Star5112. http://www.flickr.com/photos/johnjoh/463607020/. CC-BY-SA.
Jurvetson. http://www.flickr.com/photos/jurvetson/1537623/. CC-BY-SA.
A. Belani. http://www.flickr.com/photos/amitbelani/199288914/. CC-BY-SA.
http://biology.plosjournals.org/perlserv/?request=slideshow&type= figure&doi=10.1371/journal.pbio.0000008&id=39336. CC-BY.
Rama. Objective lenses of a light microscope.. CC-BY-SA France.
Sebastian Ritter. http://en.wikipedia.org/wiki/Image:Streichholz.jpg. CC–BY-SA-2.5.
NASA/JPL-Caltech/Univ. of Ariz. http://commons.wikimedia.org/wiki/Image:169141main_piaa09178.jpg. Public Domain.
Rafal Konieczny. Symbol for the eyewash fountain.. Copyrighted Free Use.
http://en.wikipedia.org/wiki/File:High_precision_Low_accuracy.svg. Public Domain.
Cnelson, Modified by: CK-12 Foundation. http://en.wikibooks.org/wiki/Image:Ap_biology_scienceofbiology01.jpg. Public Domain.
http://en.wikipedia.org/wiki/Image:Tokay_Gecko.jpg. GFDL,Public Domain.
NASA,PJLewis. http://commons.wikimedia.org/wiki/Image: Solar_sys.jpg,http://www.flickr.com/photos/pjlewis/53056038/. Public Domain,CC-BY-SA.
www.ck12.org 92
Describe elements and compounds, and explain how mixtures differ from compounds.
Define energy, and describe how energy can be changed from one form to another.
Identify three states of matter, and explain how they differ.
Living things are made of matter. In fact, matter is the “stuff” of which all things are made. Anything that occupies space and has mass is known as matter. Matter, in turn, consists of chemical substances.
A chemical substance is a material that has a definite chemical composition. It is also ho- mogeneous, so the same chemical composition is found uniformly throughout the substance. A chemical substance may be an element or a chemical compound.
An element is a pure substance that cannot be broken down into different types of sub- stances. Examples of elements include carbon, oxygen, hydrogen, and iron. Each element is made up of just one type of atom. An atom is the smallest particle of an element that still
characterizes the element. As shown in Figure 2.1, at the center of an atom is a nucleus. The nucleus contains positively charged particles called protons and electrically neutral par- ticles called neutrons. Surrounding the nucleus is a much larger electron cloud consisting of negatively charged electrons. An atom is electrically neutral if it has the same number of protons as electrons. Each element has atoms with a characteristic number of protons. For example, all carbon atoms have six protons, and all oxygen atoms have eight protons.
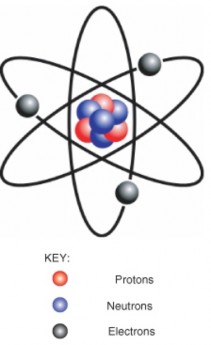
There are almost 120 known elements (Figure 2.2). The majority of known elements are classified as metals. Metals are elements that are lustrous, or shiny. They are also good conductors of electricity and heat. Examples of metals include iron, gold, and copper. Fewer than 20 elements are classified as nonmetals. Nonmetals lack the properties of metals. Examples of nonmetals include oxygen, hydrogen, and sulfur. Certain other elements have properties of both metals and nonmetals. They are known as metalloids. Examples of metalloids include silicon and boron.
www.ck12.org 94
Figure 2.2: The Periodic Table. (3)
A chemical compound is a new substance that forms when atoms of two or more elements react with one another. A chemical reaction is a process that changes some chemical sub- stances into other chemical substances. A compound that results from a chemical reaction always has a unique and fixed chemical composition. The substances in the compound can be separated from one another only by another chemical reaction. This is covered further in the Chemical Reactions lesson.
The atoms of a compound are held together by chemical bonds. Chemical bonds form when atoms share electrons. There are different types of chemical bonds, and they vary in how strongly they hold together the atoms of a compound. Two of the strongest types of bonds are covalent and ionic bonds. Covalent bonds form between atoms that have little if any difference in electronegativity. Electronegativity is the power of an atom to attract electrons toward itself. Ionic bonds, in contrast, form between atoms that are significantly different in electronegativity.
An example of a chemical compound is water. A water molecule forms when oxygen (O) and hydrogen (H) atoms react and are held together by covalent bonds. Like other compounds, water always has the same chemical composition: a 2:1 ratio of hydrogen atoms to oxygen atoms. This is expressed in the chemical formula H2O. A model of a water molecule is shown in Figure 2.3.
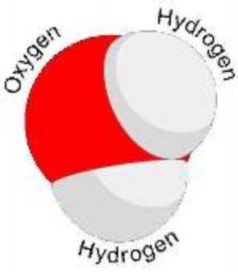
Figure 2.3: Model of a water molecule, showing the arrangement of hydrogen and oxygen atoms (17)
Compounds that contain mainly the elements carbon and hydrogen are called organic compounds. This is because they are found mainly in living organisms. Most organic compounds are held together by covalent bonds. An example of an organic compound is glucose (C6H12O6), which is shown in Figure 2.4. Glucose is a simple sugar that living cells use for energy. All other compounds are called inorganic compounds. Water is an example of an inorganic compound. You will read more about organic compounds in Lesson 2.2.
Like a chemical compound, a mixture consists of more than one chemical substance. Unlike a compound, a mixture does not have a fixed chemical composition. The substances in a mixture can be combined in any proportions. A mixture also does not involve a chemical reaction. Therefore, the substances in a mixture are not changed into unique new substances, and they can be separated from each other without a chemical reaction.
The following examples illustrate these differences between mixtures and compounds. Both examples involve the same two elements: the metal iron (Fe) and the nonmetal sulfur (S).
When iron filings and sulfur powder are mixed together in any ratio, they form a mixture. No chemical reaction occurs, and both elements retain their individual prop- erties. A magnet can be used to mechanically separate the two elements by attracting the iron filings out of the mixture and leaving the sulfur behind.
When iron and sulfur are mixed together in a certain ratio and heated, a chemical reaction occurs. This results in the formation of a unique new compound, called iron
www.ck12.org 96
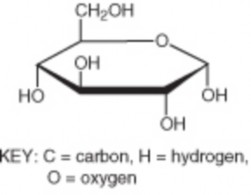
sulfide (FeS). A magnet cannot be used to mechanically separate the iron from the iron sulfide because metallic iron does not exist in the compound. Instead, another chemical reaction is required to separate the iron and sulfur.
Energy is a property of matter that is defined as the ability to do work. The concept of energy is useful for explaining and predicting most natural phenomena, and it is foundational for an understanding of biology. All living organisms need energy to grow and reproduce. However, energy can never be created or destroyed. It is always conserved. This is called the law of conservation of energy. Therefore, organisms cannot create the energy they need. Instead, they must obtain energy from the environment. Organisms also cannot destroy or use up the energy they obtain. They can only change it from one form to another.
Energy can take several different forms. Common forms of energy include light, chemical, and heat energy. Other common forms are kinetic and potential energy.
In organisms, energy is always changing from one form to another. For example, plants ob- tain light energy from sunlight and change it to chemical energy in food molecules. Chemical energy is energy stored in bonds between atoms within food molecules. When other organ- isms eat and digest the food, they break the chemical bonds and release the chemical energy. Organisms do not use energy very efficiently. About 90 percent of the energy they obtain from food is converted to heat energy that is given off to the environment.
Energy also constantly changes back and forth between kinetic and potential energy. Kinetic energy is the energy of movement. For example, a ball falling through the air has kinetic energy because it is moving (Figure 2.5). Potential energy is the energy stored in an object due to its position. A bouncing ball at the top of a bounce, just before it starts to fall, has potential energy. For that instant, the ball is not moving, but it has the potential to move because gravity is pulling on it. Once the ball starts to fall, the potential energy changes to kinetic energy. When the ball hits the ground, it gains potential energy from the impact. The potential energy changes to kinetic energy when the ball bounces back up into the air. As the ball gains height, it regains potential energy because of gravity.
www.ck12.org 98
Like the ball, every time you move you have kinetic energy — whether you jump or run or just blink your eyes. Can you think of situations in which you have potential energy? Obvious examples might include when you are standing on a diving board or at the top of a ski slope or bungee jump. What gives you potential energy in all of these situations? The answer is gravity.
The amount of energy in molecules of matter determines the state of matter. Matter can exist in one of several different states, including a gas, liquid, or solid state. These different states of matter have different properties, which are illustrated in Figure 2.6.
A gas is a state of matter in which atoms or molecules have enough energy to move freely. The molecules come into contact with one another only when they randomly collide. Forces between atoms or molecules are not strong enough to hold them to- gether.
A liquid is a state of matter in which atoms or molecules are constantly in contact but have enough energy to keep changing positions relative to one another. Forces between atoms or molecules are strong enough to keep the molecules together but not strong enough to prevent them from moving.
A solid is a state of matter in which atoms or molecules do not have enough energy to move. They are constantly in contact and in fixed positions relative to one another.
Forces between atoms or molecules are strong enough to keep the molecules together and to prevent them from moving.
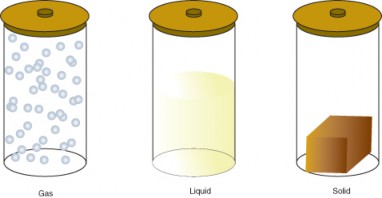
Figure 2.6: States of Matter. (4)
All three containers contain a substance with the same mass, but the substances are in different states. In the left-hand container, the substance is a gas, which has spread to fill its container. It takes both the shape and volume of the container. In the middle container, the substance is a liquid, which has spread to take the shape of its container but not the volume. In the right-hand container, the substance is a solid, which takes neither the shape nor the volume of its container.
Which state a substance is in depends partly on temperature and air pressure. For example, at the air pressure found at sea level, water exists as a liquid at temperatures between 0° C and 100° C. Above 100° C, water exists as a gas (water vapor). Below 0° C, water exists as a solid (ice). Different substances have a different range of temperatures at which they exist in each state. For example, oxygen is gas above -183° C, but iron is a gas only above 2861° C. These differences explain why some substances are always solids at normal Earth temperatures, whereas others are always gases or liquids.
Matter constantly goes through cycles that involve changing states. Water and all the elements important to organisms, including carbon and nitrogen, are constantly recycled on Earth (see Principles of Ecology). As matter moves through its cycles, it changes state repeatedly. For example, in the water cycle, water repeatedly changes from a gas to a liquid or solid and back to a gas again. How does this happen?
www.ck12.org 100
Adding energy to matter gives its atoms or molecules the ability to resist some of the forces holding them together. For example, heating ice to its melting point (0°C) gives its molecules enough energy to move. The ice melts and becomes liquid water. Similarly, heating liquid water to its boiling point (100°C) gives its molecules enough energy to pull apart from one another so they no longer have contact. The liquid water vaporizes and becomes water vapor.
Matter consists of elements and compounds. A compound forms when elements com- bine in fixed proportions and undergo a chemical reaction. A mixture forms when substances combine in any proportions without a chemical reaction.
Energy is a property of matter. It cannot be created or destroyed. Organisms obtain light energy from sunlight or chemical energy from food and change the energy into different forms, including heat energy.
Matter can exist in one of several different states, including a gas, liquid, or solid state. States of matter differ in the amount of energy their molecules have. When matter recycles, it changes state by gaining or losing energy.
Define element, and give an example of an element.
State how a compound differs from an element, and give an example of a compound.
What is energy?
What are three common states of matter?
Describe two ways that energy changes form in the following sequence of events.
A plant grows in the sun. → A rabbit eats the plant.
Describe a real-life situation in which the energy of an object or person changes back and forth between kinetic energy and potential energy. Identify each time energy changes form.
Compare and contrast mixtures and compounds.
Explain what happens to molecules of matter when matter changes state from a liquid to a gas.
David Bodanis, E = mc2: A Biography of the World’s Most Famous Question. Walker and Co., 2005.
John Emsley, Nature’s Building Blocks: An A-Z Guide to the Elements. Oxford Uni- versity Press, 2003.
Nevin Katz, Elements, Compounds, and Mixtures: Middle and High School (Mr. Bird- ley Teaches Science). Incentive Publications, 2007.
chemical compound Unique substance with a fixed composition that forms when atoms of two or more elements react.
element Pure substance made up of just one type of atom.
energy Property of matter that is defined as the ability to do work.
gas State of matter in which atoms or molecules have enough energy to move freely.
kinetic energy Form of energy that an object has when it is moving.
liquid State of matter in which atoms or molecules are constantly in contact but have enough energy to keep changing positions relative to one another.
matter All the substances of which things are made.
mixture Combination of chemical substances that does not have a fixed composition and does not result from a chemical reaction.
organic compound Type of chemical compound that contains carbon and hydrogen and is found mainly in organisms.
potential energy Form of energy that is stored in an object due to its position.
solid State of matter in which atoms or molecules do not have enough energy to move.
state of matter Condition that matter is in, depending on how much energy its atoms or molecules have.
www.ck12.org 102
Like all living things, you contain many organic compounds. For example, your brain is using the organic compound glucose as you read these words. Glucose provides brain cells with energy.
What are some other organic compounds in your body?
What roles do you think other organic compounds might play?
Why are organic compounds able to carry out these roles?
How do organic compounds differ from inorganic compounds?
Explain why carbon is essential to life on Earth.
Describe the structure and function of carbohydrates.
Describe the structure and function of lipids.
Describe the structure and function of proteins.
Describe the structure and function of nucleic acids.
Organic compounds are chemical substances that make up organisms and carry out life processes. All organic compounds contain the elements carbon and hydrogen. Because carbon is the major element in organic compounds, it is essential to all known life on Earth. Without carbon, life as we know it could not exist.
Why is carbon so important to organisms? The answer lies with carbon’s unique properties. Carbon has an exceptional ability to bind with a wide variety of other elements. Carbon atoms can form multiple stable bonds with other small atoms, including hydrogen, oxygen, and nitrogen. Carbon atoms can also form stable bonds with other carbon atoms. In fact, a carbon atom may form single, double, or even triple bonds with other carbon atoms. This allows carbon atoms to form a tremendous variety of very large and complex molecules.
Nearly 10 million carbon-containing organic compounds are known. Types of carbon com- pounds in organisms include carbohydrates, lipids, proteins, and nucleic acids. The elements found in each type are listed in Table 1. Elements other than carbon and hydrogen usually
occur within organic compounds in smaller groups of elements called functional groups. When organic compounds react with other compounds, generally just the functional groups are involved. Therefore, functional groups generally determine the nature and functions of organic compounds.
Table 2.1: Organic Compounds
![]()
Type of Compound Elements It Contains Examples
![]()
Carbohydrates Carbon, hydrogen, oxygen Glucose, Starch, Glycogen Lipids Carbon, hydrogen, oxygen Cholesterol, Triglycerides
(fats) Phospholipids
Proteins Carbon, hydrogen, oxygen, nitrogen, sulfur
Nucleic Acids Carbon, hydrogen, oxygen, nitrogen, phosphorus
Enzymes, Antibodies
Deoxyribonucleic acid (DNA) Ribonucleic acid (RNA)
![]()
This table lists the four types of organic compounds, the elements they contain, and examples of each type of compound.
Carbohydrates are organic compounds that contain only carbon, hydrogen, and oxygen. They are the most common of the four major types of organic compounds. There are thousands of different carbohydrates, but they all consist of one or more smaller units called monosaccharides.
The general formula for a monosaccharide is:
(CH2O)n,
where n can be any number greater than two. For example, if n is 6, then the formula can be written:
C6H12O6.
This is the formula for the monosaccharide glucose. Another monosaccharide, fructose, has the same chemical formula as glucose, but the atoms are arranged differently. Molecules with the same chemical formula but with atoms in a different arrangement are called iso- mers. Compare the glucose and fructose molecules in Figure 2.7. Can you identify their
www.ck12.org 104
differences? The only differences are the positions of some of the atoms. These differences affect the properties of the two monosaccharides.
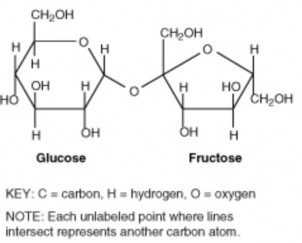
If two monosaccharides bond together, they form a carbohydrate called a disaccharide. An example of a disaccharide is sucrose (table sugar), which consists of the monosaccharides glucose and fructose (Figure 2.7). Monosaccharides and disaccharides are also called simple sugars. They provide the major source of energy to living cells.
If more than two monosaccharides bond together, they form a carbohydrate called a polysac- charide. A polysaccharide may contain anywhere from a few monosaccharides to several thousand monosaccharides. Polysaccharides are also called complex carbohydrates. Their main functions are to store energy and form structural tissues. Examples of several polysac- charides and their roles are listed in Table 2.
Table 2.2: Complex Carbohydrates
Complex Carbohydrate | Function | Organism |
Amylose | Stores energy | Plants |
Glycogen | Stores energy | Animals |
Cellulose | Forms cell walls | Plants |
Chitin | Forms external skeleton | Some animals |
These complex carbohydrates play important roles in living organisms.
Lipids are organic compounds that contain mainly carbon, hydrogen, and oxygen. They include substances such as fats and oils. Lipid molecules consist of fatty acids, with or without additional molecules. Fatty acids are organic compounds that have the general formula CH3(CH2)nCOOH, where n usually ranges from 2 to 28 and is always an even number.
Fatty acids can be saturated or unsaturated. The term saturated refers to the placement of hydrogen atoms around the carbon atoms. In a saturated fatty acid, all the carbon atoms (other than the carbon in the -COOH group) are bonded to as many hydrogen atoms as possible (usually two hydrogens). Saturated fatty acids do not contain any other groups except -COOH. This is why they form straight chains, as shown in Figure 2.8. Because of this structure, saturated fatty acids can be packed together very tightly. This allows organisms to store chemical energy very densely. The fatty tissues of animals contain mainly saturated fatty acids.
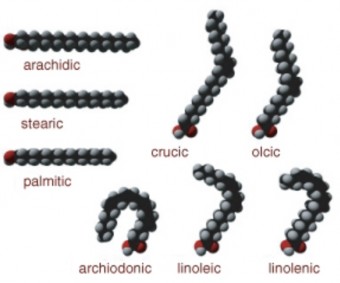
www.ck12.org 106
In an unsaturated fatty acid, some carbon atoms are not bonded to as many hydrogen atoms as possible. This is because they are bonded to one or more additional groups, including double and triple bonds between carbons. Wherever these other groups bind with carbon, they cause the chain to bend - they do not form straight chains (Figure 2.8). This gives unsaturated fatty acids different properties than saturated fatty acids. For example, unsaturated fatty acids are liquids at room temperature whereas saturated fatty acids are solids. Unsaturated fatty acids are found mainly in plants, especially in fatty tissues such as nuts and seeds.
Unsaturated fatty acids occur naturally in the bent shapes shown in Figure 2.8. However, unsaturated fatty acids can be artificially manufactured to have straight chains like saturated fatty acids. Called trans fatty acids, these synthetic lipids were commonly added to foods, until it was found that they increased the risk for certain health problems. Many food manufacturers no longer use trans fatty acids for this reason.
Lipids may consist of fatty acids alone or in combination with other compounds. Several types of lipids consist of fatty acids combined with a molecule of alcohol:
Triglycerides are the main form of stored energy in animals. This type of lipid is commonly called fat. A triglyceride is shown in Figure 2.9.
Phospholipids are a major component of the membranes surrounding the cells of all organisms.
Steroids (or sterols) have several functions. The sterol cholesterol is an important part of cell membranes and plays other vital roles in the body. Other steroids are male and female sex hormones (see Reproductive System and Human Development).
Humans need lipids for many vital functions, such as storing energy and forming cell mem- branes. Lipids can also supply cells with energy. In fact, a gram of lipids supplies more than twice as much energy as a gram of carbohydrates or proteins. Lipids are necessary in the diet for most of these functions. Although the human body can manufacture most of the lipids it needs, there are others, called essential fatty acids, that must be consumed in food. Essential fatty acids include omega-3 and omega-6 fatty acids. Both of these fatty acids are needed for important biological processes, not just for energy.
Although some lipids in the diet are essential, excess dietary lipids can be harmful. Because lipids are very high in energy, eating too many may lead to unhealthy weight gain. A high- fat diet may also increase lipid levels in the blood. This, in turn, can increase the risk for
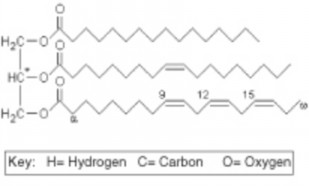
health problems such as cardiovascular disease (see Circulatory and Respiratory Systems). The dietary lipids of most concern are saturated fatty acids, trans fats, and cholesterol. For example, cholesterol is the lipid mainly responsible for narrowing arteries and causing the disease atherosclerosis.
Proteins are organic compounds that contain carbon, hydrogen, oxygen, nitrogen, and, in some cases, sulfur. Proteins are made of smaller units called amino acids. There are 20 different common amino acids needed to make proteins. All amino acids have the same basic structure, which is shown in Figure 2.10. Only the side chain (labeled R in the figure) differs from one amino acid to another. The variable side chain gives each amino acid unique properties. Proteins can differ from one another in the number and sequence (order) of amino acids. It is because of the side chains of the amino acids that proteins with different amino acid sequences have different shapes and different chemical properties.
Small proteins can contain just a few hundred amino acids. Yeast proteins average 466 amino acids. The largest known proteins are the titins, found in muscle, which are composed from almost 27,000 amino acids.
www.ck12.org 108
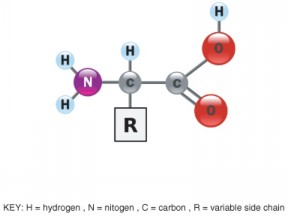
Amino acids can bond together to form short chains called peptides or longer chains called polypeptides (Figure 2.11). Polypeptides may have as few as 40 amino acids or as many as several thousand. A protein consists of one or more polypeptide chains. The sequence of amino acids in a protein’s polypeptide chain(s) determines the overall structure and chemical properties of the protein. Primary protein structure is sequence of a chain of amino acids.
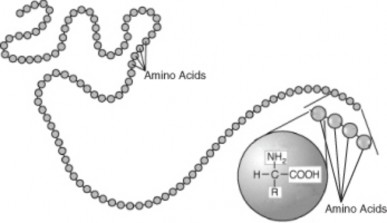
Figure 2.11: Polypeptide. This polypeptide is a chain made up of many linked amino acids. (22)
The amino acid sequence is the primary structure of a protein. As explained in Figure 2.12, a protein may have up to four levels of structure, from primary to quaternary. The complex structure of a protein allows it to carry out its biological functions.
Proteins are an essential part of all organisms. They play many roles in living things. Certain proteins provide a scaffolding that maintains the shape of cells. Proteins also make up the majority of muscle tissues. Many proteins are enzymes that speed up chemical reactions in cells (see the Chemical Reactions lesson). Other proteins are antibodies. They bond to foreign substances in the body and target them for destruction (see the Immune System and Disease chapter). Still other proteins help carry messages or materials in and out of cells or around the body. For example, the blood protein hemoglobin bonds with oxygen and carries it from the lungs to cells throughout the body.
One of the most important traits of proteins, allowing them to carry out these functions, is their ability to bond with other molecules. They can bond with other molecules very specifically and tightly. This ability, in turn, is due to the complex and highly specific structure of protein molecules.
www.ck12.org 110
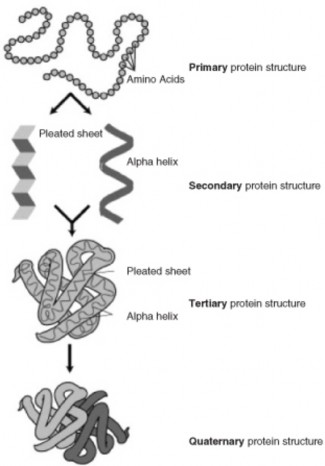
Figure 2.12: Protein Structure. Primary protein structure is the sequence of amino acids in a single polypeptide. Secondary protein structure refers to internal shapes, such as alpha helices and beta sheets, that a single polypeptide takes on due to bonds between atoms in different parts of the polypeptide. Tertiary protein structure is the overall three-dimensional shape of a protein consisting of one polypeptide. Quaternary protein structure is the shape of a protein consisting of two or more polypeptides. For a brief animation of protein structure, see www.stolaf.edu/people/giannini/flashanimat/proteins/protein%20structure.swf. (11)
Proteins in the diet are necessary for life. Dietary proteins are broken down into their component amino acids when food is digested. Cells can then use the components to build new proteins. Humans are able to synthesize all but eight of the twenty common amino acids. These eight amino acids, called essential amino acids, must be consumed in foods. Like dietary carbohydrates and lipids, dietary proteins can also be broken down to provide cells with energy.
Nucleic acids are organic compounds that contain carbon, hydrogen, oxygen, nitrogen, and phosphorus. They are made of smaller units called nucleotides. Nucleic acids are named for the nucleus of the cell, where some of them are found. Nucleic acids are found not only in all living cells but also in viruses. Types of nucleic acids include deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).
A nucleic acid consists of one or two chains of nucleotides held together by chemical bonds. Each individual nucleotide unit consists of three parts:
a base (containing nitrogen)
a sugar (ribose in RNA, deoxyribose in DNA)
a phosphate group (containing phosphorus)
The sugar of one nucleotide binds to the phosphate group of the next nucleotide. Alternating sugars and phosphate groups form the backbone of a nucleotide chain, as shown in Figure
2.13. The bases, which are bound to the sugars, stick out at right angles from the backbone of the chain.
RNA consists of a single chain of nucleotides, and DNA consists of two chains of nucleotides. Bonds form between the bases on the two chains of DNA and hold the chains together (Figure 2.13). There are four different types of bases in a nucleic acid molecule: cytosine, adenine, guanine, and either thymine (in DNA) or uracil (in RNA). Each type of base bonds with just one other type of base. Cytosine and guanine always bond together, and adenine and thymine (or uracil) always bond with one another. The pairs of bases that bond together are called complementary bases.
The binding of complementary bases allows DNA molecules to take their well-known shape, called a double helix. Figure 2.14 shows how two chains of nucleotides form a DNA double helix. A simplified double helix is illustrated in Figure 2.15. It shows more clearly
www.ck12.org 112
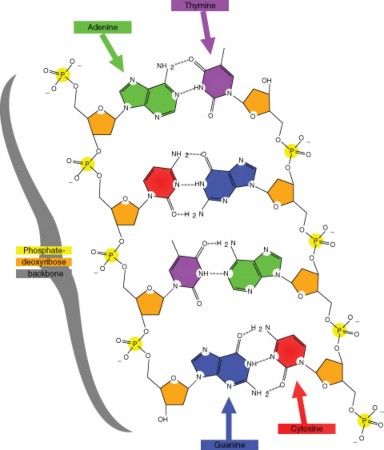
how the two chains are intertwined. The double helix shape forms naturally and is very strong. Being intertwined, the two chains are difficult to break apart. This is important given the fundamental role of DNA in all living organisms.
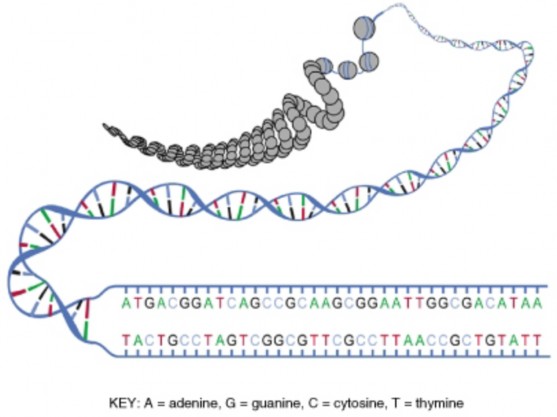
G. These bonds help maintain the double helix shape of the molecule. (25)
The order of bases in nucleic acids is highly significant. The bases are like the letters of a four-letter alphabet. These “letters” can be combined to form “words.” Groups of three bases form words of the genetic code. Each code word stands for a different amino acid. A series of many code words spells out the sequence of amino acids in a protein (Figure 2.16). In short, nucleic acids contain the information needed for cells to make proteins. This
www.ck12.org 114

information is passed from a body cell to its daughter cells when the cell divides. It is also passed from parents to their offspring when organisms reproduce.
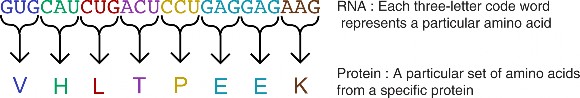
DNA and RNA have different functions relating to the genetic code and proteins. Like a set of blueprints, DNA contains the genetic instructions for the correct sequence of amino acids in proteins. RNA uses the information in DNA to assemble the amino acids and make the proteins. You will read more about the genetic code and the role of nucleic acids in protein synthesis in Chapter 8.
Carbon’s exceptional ability to form bonds with other elements and with itself allows it to form a huge number of large, complex molecules called organic molecules. These molecules make up organisms and carry out life processes.
Carbohydrates are organic molecules that consist of carbon, hydrogen, and oxygen. They are made up of repeating units called saccharides. They provide cells with energy, store energy, and form structural tissues.
Lipids are organic compounds that consist of carbon, hydrogen, and oxygen. They are made up of fatty acids and other compounds. They provide cells with energy, store energy, and help form cell membranes.
Proteins are organic compounds that consist of carbon, hydrogen, oxygen, nitrogen, and, in some cases, sulfur. They are made up of repeating units called amino acids. They provide cells with energy, form tissues, speed up chemical reactions throughout the body, and perform many other cellular functions.
Nucleic acids are organic compounds that consist of carbon, hydrogen, oxygen, nitro- gen, and phosphorus. They are made up of repeating units called nucleotides. They contain genetic instructions for proteins, help synthesize proteins, and pass genetic instructions on to daughter cells and offspring.
State the function of monosaccharides, such as glucose and fructose.
Why do molecules of saturated and unsaturated fatty acids have different shapes?
What determines the primary structure of a protein?
Identify the three parts of a nucleotide.
What type of organic compound is represented by the formula CH3(CH2)4COOH? How do you know?
Bases in nucleic acids are represented by the letters A, G, C, and T (or U). How are the bases in nucleic acids like the letters of an alphabet.
Why is carbon essential to all known life on Earth?
Compare and contrast simple sugars and complex carbohydrates.
State two functions of proteins, and explain how the functions depend on the ability of proteins to bind other molecules to them.
B.G. Davis and A.J. Fairbanks, Carbohydrate Chemistry. Oxford University Press, 2002.
Michael I. Gurr, John L. Harwood, and Keith N. Frayn, Lipid Biochemistry: An Introduction. Wiley, 2005.
www.ck12.org 116
James D. Watson, The Double Helix: A Personal Account of the Discovery of DNA. Touchstone, 2001.
David Whitford, Proteins: Structure and Functions. Wiley, 2005.
amino acid Small organic molecule that is a building block of proteins.
carbohydrate Type of organic compound that consists of one or more smaller units called monosaccharides.
cholesterol Type of steroid that is an important part of cell membranes and plays other vital roles.
complementary bases Nucleic acid bases that form bonds with each other and help hold together two nucleotide chains.
complex carbohydrate Another term for a polysaccharide.
deoxyribonucleic acid (DNA) Double-stranded nucleic acid that contains the genetic instructions for proteins.
disaccharide Small carbohydrate, such as sucrose, that consists of two monosaccharides.
double helix Normal shape of a DNA molecule in which two chains of nucleotides are intertwined.
essential amino acids Amino acids that the human body needs but cannot make and must consume in food.
essential fatty acids Fatty acids that the human body needs but cannot make and must consume in food.
fatty acid Organic compound found in lipids that has the general formula CH3(CH2)nCOOH.
functional group Small group of elements within an organic compound that determines the nature and function of the organic compound.
lipid Type of organic compound that consists of one or more fatty acids with or without additional molecules.
monosaccharide Small carbohydrate, such as glucose, with the general formula (CH2O)n. nucleic acid Type of organic compound that consists of smaller units called nucleotides. nucleotide Small organic molecule that is a building block of nucleic acids.
peptide Short chain of amino acids.
phospholipid Type of lipid that is a major component of cell membranes.
polypeptide Long chain of amino acids.
polysaccharide Large carbohydrate that consists of more than two monosaccharides. protein Type of organic compound that consists of smaller units called amino acids. ribonucleic acid (RNA) Single-stranded nucleic acid that uses information contained in
DNA to assemble amino acids and make proteins.
saturated fatty acid Type of fatty acid in which all the carbon atoms are bonded to as many hydrogen atoms as possible.
simple sugar Another term for a monosaccharide or disaccharide.
steroid Type of lipid that has several functions, such as forming cell membranes and acting as sex hormones.
trans fatty acid Artificial, unsaturated fatty acid that has properties similar to saturated fatty acids.
triglyceride Type of lipid that is the main form of stored energy in animals.
unsaturated fatty acid Type of fatty acid in which some carbon atoms are not bonded to as many hydrogen atoms as possible.
www.ck12.org 118
Organisms are made up of thousands of very large, complex molecules called organic molecules. These molecules consist of repeating units of smaller molecules, such as amino acids or nu- cleotides.
How do organic molecules form?
How do smaller molecules join together to form larger molecules?
What chemical processes are involved?
Describe what happens in a chemical reaction, and identify types of chemical reactions.
Explain the role of energy in chemical reactions, and define activation energy.
State factors that affect the rate of chemical reactions.
Explain the importance of enzymes in organisms, and describe how enzymes work.
A chemical compound may be very different from the substances that combine to form it. For example, the element chlorine (Cl) is a poisonous gas, but when it combines with sodium (Na) to form sodium chloride (NaCl), it is no longer toxic. You may even eat it on your food. Sodium chloride is just table salt. What process changes a toxic chemical like chlorine into a much different substance like table salt?
A chemical reaction is a process that changes some chemical substances into other chem- ical substances. The substances that start a chemical reaction are called reactants. The substances that form as a result of a chemical reaction are called products. During the re- action, the reactants are used up to create the products. For example, when methane burns in oxygen, it releases carbon dioxide and water. In this reaction, the reactants are methane (CH4) and oxygen (O2), and the products are carbon dioxide (CO2) and water (H2O).
A chemical reaction can be represented by a chemical equation. Using the same example, the burning of methane gas can be represented by the equation:
CH4 + 2 O2 → CO2 + 2 H2O.
The arrow in a chemical equation separates the reactants from the products and shows the direction in which the reaction occurs. If the reaction could also occur in the opposite direction, then two arrows, one pointing in each direction, would be used. On each side of the arrow, a mixture of chemicals is indicated by the chemical symbols joined by a plus sign (+). The numbers preceding some of the chemical symbols (such as 2 O2) indicate how many molecules of the chemicals are involved in the reaction. (If there is no number in front of a chemical symbol, it means that just one molecule is involved.)
In a chemical reaction, the quantity of each element does not change. There is the same amount of each element at the end of the reaction as there was at the beginning. This is reflected in the chemical equation for the reaction. The equation should be balanced. In a balanced equation, the same number of atoms of a given element appear on each side of the arrow. For example, in the equation above, there are four hydrogen atoms on each side of the arrow.
In general, a chemical reaction involves the breaking and forming of chemical bonds. In the methane reaction above, bonds are broken in methane and oxygen, and bonds are formed in carbon dioxide and water. A reaction like this, in which a compound or element burns in oxygen, is called a combustion reaction. This is just one of many possible types of chemical reactions. Other types of chemical reactions include synthesis, decomposition, and substitution reactions.
A synthesis reaction occurs when two or more chemical elements or compounds unite to form a more complex product. For example, nitrogen (N2) and hydrogen (H2) unite to form ammonia (NH3):
N2 + 3 H2 → 2 NH3.
A decomposition reaction occurs when a compound is broken down into smaller compounds or elements. For example, water (H2O) breaks down into hydrogen (H2) and oxygen (O2):
2 H2O → 2 H2 + O2.
A substitution reaction occurs when one element replaces another element in a compound. For example, sodium (Na+) replaces hydrogen (H) in hydrochloric acid (HCl), producing sodium chloride (NaCl) and hydrogen gas (H2):
2 Na+ + 2 HCl → 2 NaCl + H2.
www.ck12.org 120
Some chemical reactions consume energy, whereas other chemical reactions release energy. Each of the energy changes that occur during a reaction are graphed in Figure 2.17. In the reaction on the left, energy is released. In the reaction on the right, energy is consumed.
Figure 2.17: The reaction on the left releases energy. The reaction on the right consumes energy. (23)
Chemical reactions that release energy are called exothermic reactions. An example is the combustion of methane described at the beginning of this lesson. In organisms, exothermic reactions are called catabolic reactions. Catabolic reactions break down molecules into smaller units. An example is the breakdown of glucose molecules for energy. Exothermic reactions can be represented by the general chemical equation:
Reactants → Products + Heat.
Chemical reactions that consume energy are called endothermic reactions. An example is the synthesis of ammonia, described above. In organisms, endothermic reactions are called anabolic reactions. Anabolic reactions construct molecules from smaller units. An example is the synthesis of proteins from amino acids. Endothermic reactions can be represented by the general chemical equation:
Reactants + Heat → Products.
Regardless of whether reactions are exothermic or endothermic, they all need energy to get started. This energy is called activation energy. Activation energy is like the push you need to start moving down a slide. The push gives you enough energy to start moving. Once you start, you keep moving without being pushed again. The concept of activation energy is illustrated in Figure 2.18.
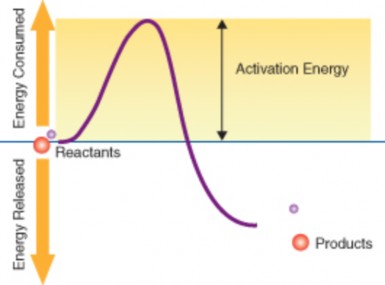
Why do reactions need energy to get started? In order for reactions to occur, three things must happen, and they all require energy:
Reactant molecules must collide. To collide, they must move, so they need kinetic energy.
Unless reactant molecules are positioned correctly, intermolecular forces may push them apart. To overcome these forces and move together requires more energy.
If reactant molecules collide and move together, there must be enough energy left for them to react.
www.ck12.org 122
The rates at which chemical reactions take place in organisms are very important. Chemical reactions in organisms are involved in processes ranging from the contraction of muscles to the digestion of food. For example, when you wave goodbye, it requires repeated contractions of muscles in your arm over a period of a couple of seconds. A huge number of reactions must take place in that time, so each reaction cannot take longer than a few milliseconds. If the reactions took much longer, you might not finish waving until sometime next year.
Factors that help reactant molecules collide and react speed up chemical reactions. These factors include the concentration of reactants and the temperature at which the reactions occur.
Reactions are usually faster at higher concentrations of reactants. The more reactant molecules there are in a given space, the more likely they are to collide and react.
Reactions are usually faster at higher temperatures. Reactant molecules at higher temperatures have more energy to move, collide, and react.
Most chemical reactions within organisms would be impossible under the conditions in cells. For example, the body temperature of most organisms is too low for reactions to occur quickly enough to carry out life processes. Reactants may also be present in such low concentrations that it is unlikely they will meet and collide. Therefore, the rate of most biochemical reactions must be increased by a catalyst. A catalyst is a chemical that speeds up chemical reactions. In organisms, catalysts are called enzymes.
Like other catalysts, enzymes are not reactants in the reactions they control. They help the reactants interact but are not used up in the reactions. Instead, they may be used over and over again. Unlike other catalysts, enzymes are usually highly specific for particular chemical reactions. They generally catalyze only one or a few types of reactions.
Enzymes are extremely efficient in speeding up reactions. They can catalyze up to several million reactions per second. As a result, the difference in rates of biochemical reactions with and without enzymes may be enormous. A typical biochemical reaction might take hours or even days to occur under normal cellular conditions without an enzyme but less than a second with the enzyme. For an animation of a reaction in the presence or absence of an enzyme, see http://www.stolaf.edu/people/giannini/flashanimat/enzymes/prox-orien.swf.
How do enzymes speed up biochemical reactions so dramatically? Like all catalysts, en- zymes work by lowering the activation energy of chemical reactions. This is illustrated in Figure 2.19. The biochemical reaction shown in the figure requires about three times as much activation energy without the enzyme as it does with the enzyme. An animation of this process can be viewed at http://www.stolaf.edu/people/giannini/flashanimat/ enzymes/transition%20state.swf.
Enzymes generally lower activation energy by reducing the energy needed for reactants to come together and react. For example:
Enzymes bring reactants together so they don’t have to expend energy moving about until they collide at random. Enzymes bind both reactant molecules (called substrate), tightly and specifically, at a site on the enzyme molecule called the active site (Figure 2.20).
www.ck12.org 124
By binding reactants at the active site, enzymes also position reactants correctly, so they do not have to overcome intermolecular forces that would otherwise push them apart. This allows the molecules to interact with less energy.
Enzymes may also allow reactions to occur by different pathways that have lower activation energy.
The activities of enzymes also depend on the temperature, ionic conditions, and the pH of the surroundings.
Some enzymes work best at acidic pHs, while others work best in neutral environments.
Digestive enzymes secreted in the acidic environment (low pH) of the stomach help break down proteins into smaller molecules. The main digestive enzyme in the stomach is pepsin, which works best at a pH of about 1.5 (see the Digestive and Excretory Systems chapter). These enzymes would not work optimally at other pHs. Trypsin is another enzyme in the digestive system which break protein chains in the food into smaller parts. Trypsin works in the small intestine, which is not an acidic environment. Trypsin’s optimum pH is about 8.
Biochemical reactions are optimal at physiological temperatures. For example, most biochemical reactions work best at the normal body temperature of 98.6˚F. Many enzymes lose function at lower and higher temperatures. At higher temperatures, an enzyme’s shape deteriorates and only when the temperature comes back to normal does the enzyme regain its shape and normal activity.
Enzymes are involved in most of the chemical reactions that take place in organisms. About 4,000 such reactions are known to be catalyzed by enzymes, but the number may be even higher. Needed for reactions that regulate cells, enzymes allow movement, transport mate- rials around the body, and move substances in and out of cells.
In animals, another important function of enzymes is to help digest food. Digestive enzymes speed up reactions that break down large molecules of carbohydrates, proteins, and fats into smaller molecules the body can use (See Chapter: Digestive and Excretory Systems). Without digestive enzymes, animals would not be able to break down food molecules quickly enough to provide the energy and nutrients they need to survive.
A chemical reaction is a process that changes some chemical substances into others. It involves breaking and forming chemical bonds. Types of chemical reactions include
www.ck12.org 126
synthesis reactions and decomposition reactions.
Some chemical reactions are exothermic, which means they release energy. Other chemical reactions are endothermic, which means they consume energy. All chemical reactions require activation energy, which is the energy needed to get a reaction started.
Rates of chemical reactions depend on factors such as the concentration of reactants and the temperature at which reactions occur. Both factors affect the ability of reactant molecules to react.
Enzymes are needed to speed up chemical reactions in organisms. They work by lowering the activation energy of reactions.
Identify the roles of reactants and products in a chemical reaction.
What is the general chemical equation for an endothermic reaction?
State two factors, other than enzymes, that speed up chemical reactions.
How do enzymes work to speed up chemical reactions?
What is wrong with the chemical equation below? How could you fix it? CH4 + O2
→ CO2 + 2 H2O
What type of reaction is represented by the following chemical equation? Explain your answer. 2 Na + 2 HCL → 2 NaCl + H2
Why do all chemical reactions require activation energy?
Explain why organisms need enzymes to survive.
Peter Atkins and Julio De Paula, Physical Chemistry for the Life Sciences. Oxford University Press, 2006.
Rita Elkins, Digestive Enzymes. Woodland Publishing, 2007.
James Keeler and Peter Wothers, Why Chemical Reactions Happen. Oxford University Press, 2003.
George W. Roberts, Chemical Reactions and Chemical Reactors. Wiley, 2008.
http://www.stolaf.edu/people/giannini/flashanimat
activation energy Energy needed for a chemical reaction to get started.
anabolic reaction Endothermic reaction that occurs in organisms.
catabolic reaction Exothermic reaction that occurs in organisms.
chemical reaction Process that changes some chemical substances into other chemical substances.
combustion reaction Type of chemical reaction in which a compound or element burns in oxygen.
decomposition reaction Type of chemical reaction in which a compound is broken down into smaller compounds or elements.
endothermic reaction Any chemical reaction that consumes energy. enzyme Chemical that speeds up chemical reactions in organisms. exothermic reaction Any chemical reaction that releases energy. product Substance that forms as a result of a chemical reaction.
reactant Substance involved in a chemical reaction that is present at the beginning of the reaction.
substitution reaction Type of chemical reaction in which one element replaces another element in a compound.
synthesis reaction Type of chemical reaction in which elements or compounds unite to form a more complex product.
Most chemical reactions in organisms take place in an environment that is mostly water.
What do you know about water?
Are you aware that water has unique properties?
Do you know how water behaves differently from most other substances on Earth?
Do you know why water is necessary for life? www.ck12.org 128
Describe the distribution of Earth’s water, and outline the water cycle.
Identify the chemical structure of water, and explain how it relates to water’s unique properties.
Define solution, and describe water’s role as a solvent.
State how water is used to define acids and bases, and identify the pH ranges of acids and bases.
Explain why water is essential for life processes.
Water, like carbon, has a special role in biology because of its importance to organisms. Water is essential to all known forms of life. Water, H2O, such a simple molecule, yet it is this simplicity that gives water its unique properties and explains why water is so vital for life.
Water is a common chemical substance on Earth. The term water generally refers to its liquid state. Water is a liquid over a wide range of standard temperatures and pressures. However, water can also occur as a solid (ice) or gas (water vapor).
Of all the water on Earth, about two percent is stored underground in spaces between rocks. A fraction of a percent exists in the air as water vapor, clouds, or precipitation. Another fraction of a percent occurs in the bodies of plants and animals. So where is most of Earth’s water? It’s on the surface of the planet. In fact, water covers about 70 percent of Earth’s surface. Of water on Earth’s surface, 97 percent is salt water, mainly in the ocean. Only 3 percent is freshwater. Most of the freshwater is frozen in glaciers and polar ice caps. The remaining freshwater occurs in rivers, lakes, and other freshwater features.
Although clean freshwater is essential to human life, in many parts of the world it is in short supply. The amount of freshwater is not the issue. There is plenty of freshwater to go around, because water constantly recycles on Earth. However, freshwater is not necessarily located where it is needed, and clean freshwater is not always available.
Like other matter on Earth, water is continuously recycled. Individual water molecules are always going through the water cycle (see the Principles of Ecology chapter). In fact, water molecules on Earth have been moving through the water cycle for billions of years. In this cycle, water evaporates from Earth’s surface (or escapes from the surface in other ways), forms clouds, and falls back to the surface as precipitation. This cycle keeps repeating. Several processes change water from one state to another during the water cycle. They include:
Evaporation—Liquid water on Earth’s surface changes into water vapor in the atmo- sphere.
Sublimation—Snow or ice on Earth’s surface changes directly into water vapor in the atmosphere.
Transpiration—Plants give off liquid water, most of which evaporates into the atmo- sphere.
Condensation—Water vapor in the atmosphere changes to liquid water droplets, forming clouds or fog.
Precipitation—Water droplets in clouds are pulled to Earth’s surface by gravity, forming rain, snow, or other type of falling moisture.
You are probably already familiar with many of water’s properties. For example, you no doubt know that water is tasteless, odorless, and transparent. In small quantities, it is also colorless. However, when a large amount of water is observed, as in a lake or the ocean, it is actually light blue in color. These and other properties of water depend on its chemical structure.
The transparency of water is important for organisms that live in water. Because water is transparent, sunlight can pass through it. Sunlight is needed by water plants and other water organisms for photosynthesis (see Biomes, Ecosystems, and Communities chapter).
www.ck12.org 130
Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical formula H2O. The arrangement of atoms in a water molecule, shown in Figure 2.21, explains many of water’s chemical properties. In each water molecule, the nucleus of the oxygen atom (with 8 positively charged protons) attracts electrons much more strongly than do the hydrogen nuclei (with only one positively charged proton). This results in a negative electrical charge near the oxygen atom (due to the ”pull” of the negatively charged electrons toward the oxygen nucleus) and a positive electrical charge near the hydrogen atoms. A difference in electrical charge between different parts of a molecule is called polarity. A polar molecule is a molecule in which part of the molecule is positively charged and part of the molecule is negatively charged.
Opposite electrical charges attract one another other. Therefore, the positive part of one water molecule is attracted to the negative parts of other water molecules. Because of this attraction, bonds form between hydrogen and oxygen atoms of adjacent water molecules, as demonstrated in Figure 2.22. This type of bond always involves a hydrogen atom, so it is called a hydrogen bond. Hydrogen bonds are bonds between molecules, and they are not as strong as bonds within molecules. Nonetheless, they help hold water molecules together.
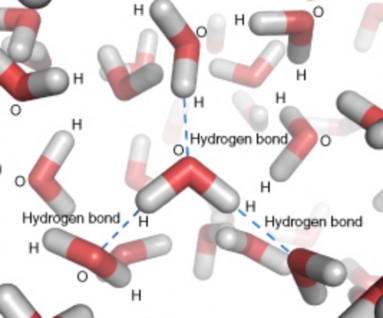
Hydrogen bonds can also form within a single large organic molecule (see the Organic Com- pounds lesson). For example, hydrogen bonds that form between different parts of a protein molecule bend the molecule into a distinctive shape, which is important for the protein’s functions. Hydrogen bonds also hold together the two nucleotide chains of a DNA molecule.
Water has some unusual properties due to its hydrogen bonds. One property is the tendency for water molecules to stick together. For example, if you drop a tiny amount of water onto a very smooth surface, the water molecules will stick together and form a droplet, rather than spread out over the surface. The same thing happens when water slowly drips from a leaky faucet. The water doesn’t fall from the faucet as individual water molecules but as droplets of water. The tendency of water to stick together in droplets is also illustrated by the dew drops in Figure 2.23.
Hydrogen bonds also explain why water’s boiling point (100° C) is higher than the boiling points of similar substances without hydrogen bonds. Because of water’s relatively high boiling point, most water exists in a liquid state on Earth. Liquid water is needed by all living organisms. Therefore, the availability of liquid water enables life to survive over much of the planet.
www.ck12.org 132

The melting point of water is 0° C. Below this temperature, water is a solid (ice). Unlike most chemical substances, water in a solid state has a lower density than water in a liquid state. This is because water expands when it freezes. Again, hydrogen bonding is the reason. Hydrogen bonds cause water molecules to line up less efficiently in ice than in liquid water. As a result, water molecules are spaced farther apart in ice, giving ice a lower density than liquid water. A substance with lower density floats on a substance with higher density. This explains why ice floats on liquid water, whereas many other solids sink to the bottom of liquid water.
In a large body of water, such as a lake or the ocean, the water with the greatest density always sinks to the bottom. Water is most dense at about 4° C. As a result, the water at the bottom of a lake or the ocean usually has temperature of about 4° C. In climates with cold winters, this layer of 4° C water insulates the bottom of a lake from freezing temperatures. Lake organisms such as fish can survive the winter by staying in this cold, but unfrozen, water at the bottom of the lake.
Water is one of the most common ingredients in solutions. A solution is a homogeneous mixture composed of two or more substances. In a solution, one substance is dissolved in another substance, forming a mixture that has the same proportion of substances throughout. The dissolved substance in a solution is called the solute. The substance in which is it
dissolved is called the solvent. An example of a solution in which water is the solvent is salt water. In this solution, a solid—sodium chloride—is the solute. In addition to a solid dissolved in a liquid, solutions can also form with solutes and solvents in other states of matter. Examples are given in Table 1.
Table 2.3:
![]()
![]()
Solvent Gas Liquid Solid Gas Oxygen and other
gases in nitrogen (air)
Liquid Carbon dioxide in water (carbonated water)
Ethanol (an alcohol) in water
Sodium chloride in water (salt water)
Solid Hydrogen in metals Mercury in silver and other metals (dental fillings)
Iron in carbon (steel)
![]()
(Source: http://en.wikipedia.org/wiki/Solute, License: Creative Commons)
The ability of a solute to dissolve in a particular solvent is called solubility. Many chemical substances are soluble in water. In fact, so many substances are soluble in water that water is called the universal solvent. Water is a strongly polar solvent, and polar solvents are better at dissolving polar solutes. Many organic compounds and other important biochemicals are polar, so they dissolve well in water. On the other hand, strongly polar solvents like water cannot dissolve strongly nonpolar solutes like oil. Did you ever try to mix oil and water? Even after being well shaken, the two substances quickly separate into distinct layers.
Water is the solvent in solutions called acids and bases. To understand acids and bases, it is important to know more about pure water, in which nothing is dissolved. In pure water (such as distilled water), a tiny fraction of water molecules naturally breaks down, or dissociates, to form ions. An ion is an electrically charged atom or molecule. The dissociation of pure water into ions is represented by the chemical equation:
2 H2O → H3O+ + OH-.
The products of this reaction are a hydronium ion (H3O+) and a hydroxide ion (OH-). The hydroxide ion is negatively charged. It forms when a water molecule donates, or gives up, a positively charged hydrogen ion. The hydronium ion, modeled in Figure 2.24, is positively charged. It forms when a water molecule accepts a positively charged hydrogen ion (H+).
www.ck12.org 134
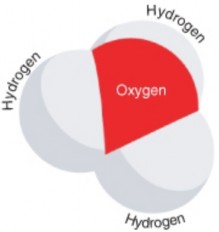
Figure 2.24: A hydronium ion has the chemical formula H3O+. The plus sign (+) indicates that the ion is positively charged. How does this molecule differ from the water molecule in Figure 2.21? (8)
Acidity refers to the hydronium ion concentration of a solution. It is measured by pH. In pure water, the hydronium ion concentration is very low. Only about one in ten million water molecules naturally dissociates to form a hydronium ion in pure water. This gives water a pH of 7. The hydronium ions in pure water are also balanced by hydroxide ions, so pure water is neutral (neither an acid nor a base).
Because pure water is neutral, any other solution with the same hydronium ion concentration and pH is also considered to be neutral. If a solution has a higher concentration of hydronium ions and lower pH than pure water, it is called an acid. If a solution has a lower concentration of hydronium ions and higher pH than pure water, it is called a base. Several acids and bases and their pH values are identified on the pH scale, which ranges from 0 to 14, in Figure 2.25.
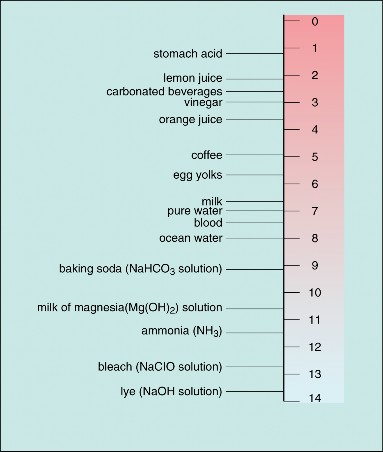
Figure 2.25: Acidity and the pH Scale Water has a pH of 7, so this is the point of neutrality on the pH scale. Acids have a pH less than 7, and bases have a pH greater than 7. (1)
www.ck12.org 136
The pH scale is a negative logarithmic scale. Because the scale is negative, as the ion concentration increases, the pH value decreases. In other words, the more acidic the solution, the lower the pH value. Because the scale is logarithmic, each one-point change in pH reflects a ten-fold change in the hydronium ion concentration and acidity. For example, a solution with a pH of 6 is ten times as acidic as pure water with a pH of 7.
An acid can be defined as a hydrogen ion donor. The hydrogen ions bond with water molecules, leading to a higher concentration of hydronium ions than in pure water. For example, when hydrochloric acid (HCl) dissolves in pure water, it donates hydrogen ions (H+) to water molecules, forming hydronium ions (H3O+) and chloride ions (Cl-). This is represented by the chemical equation:
HCl + H2O → Cl- + H3O+.
Strong acids can be harmful to organisms and damaging to materials. Acids have a sour taste and may sting or burn the skin. Testing solutions with litmus paper is an easy way to identify acids. Acids turn blue litmus paper red.
A base can be defined as a hydrogen ion acceptor. It accepts hydrogen ions from hydronium ions, leading to a lower concentration of hydronium ions than in pure water. For example, when the base ammonia (NH3) dissolves in pure water, it accepts hydrogen ions (H+) from hydronium ions (H3O+) to form ammonium ions (NH4+) and hydroxide ions (OH-). This is represented by the chemical equation:
NH3 + H2O → NH4+ + OH-.
Like strong acids, strong bases can be harmful to organisms and damaging to materials. Bases have a bitter taste and feel slimy to the touch. They can also burn the skin. Bases, like acids, can be identified with litmus paper. Bases turn red litmus paper blue.
What do you think would happen if you mixed an acid and a base? If you think the acid and base would “cancel each other out,” you are right. When an acid and base react, they form a neutral solution of water and a salt (a molecule composed of a positive and negative ion). This type of reaction is called a neutralization reaction. For example, when the base sodium hydroxide (NaOH) and hydrochloric acid (HCl) react, they form a neutral solution of water and the salt sodium chloride (NaCl). This reaction is represented by the chemical equation:
NaOH + HCl → NaCl + H2O.
In this reaction, hydroxide ions (OH-) from the base combine with hydrogen ions (H+) from the acid to form water. The other ions in the solution (Na+) and (Cl-) combine to form sodium chloride.
Enzymes are needed to speed up biochemical reactions. Most enzymes require a specific range of pH in order to do their job. For example, the enzyme pepsin, which helps break down proteins in the human stomach, requires a very acidic environment in order to function. Strong acid is secreted into the stomach, allowing pepsin to work. Once the contents of the stomach enter the small intestine, where most digestion occurs, the acid must be neutralized. This is because enzymes that work in the small intestine need a basic environment. An organ near the small intestine, called the pancreas, secretes bicarbonate ions (HCO3-) into the small intestine to neutralize the stomach acid.
Bicarbonate ions play an important role in neutralizing acids throughout the body. Bicar- bonate ions are especially important for protecting tissues of the central nervous system from changes in pH. The central nervous system includes the brain, which is the body’s control center. If pH deviates too far from normal, the central nervous system cannot function properly. This can have a drastic effect on the rest of the body.
Humans are composed of about 70 percent water (not counting water in body fat). This water is crucial for normal functioning of the body. Water’s ability to dissolve most biologically significant compounds—from inorganic salts to large organic molecules—makes it a vital solvent inside organisms and cells.
Water is an essential part of most metabolic processes within organisms. Metabolism is the sum total of all body reactions, including those that build up molecules (anabolic reactions) and those that break down molecules (catabolic reactions). In anabolic reactions, water is generally removed from small molecules in order to make larger molecules. In catabolic reactions, water is used to break bonds in larger molecules in order to make smaller molecules.
Water is central to two related, fundamental metabolic reactions in organisms: photosynthe- sis (Photosynthesis chapter) and respiration (Cellular Respiration chapter). All organisms depend directly or indirectly on these two reactions.
In photosynthesis, cells use the energy in sunlight to change water and carbon dioxide into glucose and oxygen. This is an anabolic reaction, represented by the chemical equation:
www.ck12.org 138
6 CO2 + 6 H2O + energy → C6H12O6, + 6 O2.
In cellular respiration, cells break down glucose in the presence of oxygen and release energy, water, and carbon dioxide. This is a catabolic reaction, represented by the chemical equation:
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + energy
Two other types of reactions that occur in organisms and involve water are dehydration and hydration reactions. A dehydration reaction occurs when molecules combine to form a single, larger molecule and also a molecule of water. (If some other small molecule is formed instead of water, the reaction is called by the more general term, condensation reaction.) It is a type of catabolic reaction. An example of a dehydration reaction is the formation of peptide bonds between amino acids in a polypeptide chain. When two amino acids bond together, a molecule of water is lost. This is shown in Figure 2.26.
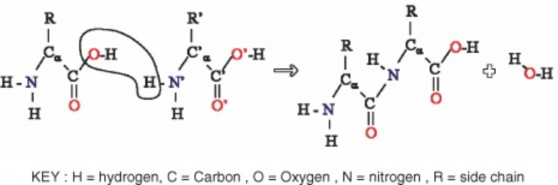
A hydration reaction is the opposite of a dehydration reaction. A hydration reaction adds water to an organic molecule and breaks the large molecule into smaller molecules. Hydration reactions occur in an acidic water solution. An example of hydration reaction is the breaking of peptide bonds in polypeptides. A hydroxide ion (OH-) and a hydrogen ion (H+) (both from a water molecule) bond to the carbon atoms that formed the peptide bond. This breaks the peptide bond and results in two amino acids.
Water is essential for all of these important chemical reactions in organisms. As a result, virtually all life processes depend on water. Clearly, without water, life as we know it could not exist.
Most of Earth’s water is salt water located on the planet’s surface. Water is constantly recycled through the water cycle.
Water molecules are polar, so they form hydrogen bonds. This gives water unique properties, such as a relatively high boiling point.
A solution is a homogeneous mixture in which a solute dissolves in a solvent. Water is a very common solvent, especially in organisms.
The ion concentration of neutral, pure water gives water a pH of 7 and sets the standard for defining acids and bases. Acids have a pH lower than 7, and bases have a pH higher than 7.
Water is essential for most life processes, including photosynthesis, cellular respiration, and other important chemical reactions that occur in organisms.
Where is most of Earth’s water?
What is polarity, and why is water polar?
Define solution, and give an example of a solution.
What is the pH of a neutral solution? Why?
Draw a circle diagram to represent the water cycle. Identify the states of water and the processes in which water changes state throughout the cycle.
What type of reaction is represented by the chemical equation below? Defend your answer. KOH + HCl → KCl + H2O
Explain how hydrogen bonds cause molecules of liquid water to stick together.
Summarize how metabolism in organisms depends on water.
Philip Ball, Life’s Matrix: A Biography of Water. University of California Press, 2001.
Robert A. Copeland, Enzymes: A Practical Introduction to Structure, Mechanisms, and Data Analysis. Wiley, 2000.
Peter Swanson, Water: The Drop of Life. Cowles Creative Publishing, 2001.
http://en.wikibooks.org/wiki/Organic_Chemistry/Introduction_to_reactions/ Alkyne_hydration
www.ck12.org 140
acid Solution with a higher hydronium ion concentration than pure water and a pH lower than 7.
acidity Hydronium ion concentration of a solution.
base Solution with a lower hydronium ion concentration than pure water and a pH higher than 7.
condensation Process in which water vapor changes to water droplets, forming clouds or fog.
evaporation Process in which liquid water changes into water vapor.
hydrogen bond Bond that forms between a hydrogen atom in one molecule and a different atom in another molecule.
ion Electrically charged atom or molecule.
metabolism Sum total of all body reactions, including those that build up molecules (anabolic reactions) and those that break down molecules (catabolic reactions).
neutralization Chemical reaction in which an acid and a base react to form a neutral solution of water and a salt.
pH Measure of the acidity, or hydronium ion concentration, of a solution. polarity Difference in electrical charge between different parts of a molecule. precipitation Rain, snow, sleet, or other type of moisture that falls from clouds. solubility Ability of a solute to dissolve in a particular solvent.
solute Substance in a solution that is dissolved by the other substance (the solvent). solution Homogeneous mixture in which one substance is dissolved in another. solvent Substance in a solution that dissolves the other substance (the solute). sublimation Process in which snow or ice changes directly into water vapor.
transpiration Process in which plants give off water, most of which evaporates.
Most life processes take place within cells. You probably know that cells are the microscopic building blocks of organisms.
What do you think you would see if you could look inside a cell?
What structures might you see?
What processes might you observe?
CK-12 Foundation. http://commons.wikimedia.org/wiki/File:PH_scale.png. CC-BY-SA.
http://en.wikipedia.org/wiki/Image:DNA_chemical_structure.svg. GNU FDL.
CK-12 Foundation. The Periodic Table.. Public Domain.
CK-12 Foundation. States of Matter. CC-BY-SA.
http://commons.wikimedia.org/wiki/Image:Glucose.png. Creative Commons.
http://en.wikipedia.org/wiki/Image:Bouncing_ball_strobe_edit.jpg. Creative Commons.
http://commons.wikimedia.org/wiki/File:Hydronium.png. Public Domain.
http://en.wikipedia.org/wiki/Image:Double_Helix.png. Public Domain.
http://en.wikipedia.org/wiki/Image:Protein-structure.png. Public Domain.
http://en.wikipedia.org/wiki/Image:Liquid_water_hydrogen_bond.png. GNU-FDL.
http://commons.wikimedia.org/wiki/Image: Fat_triglyceride_shorthand_formula.PNG. Pubic Domain.
http://en.wikipedia.org/wiki/Image:Activation2_updated.svg. GNU-FDL.
http://en.wikipedia.org/wiki/Image:Saccharose.svg. Creative Commons. www.ck12.org 142
http://commons.wikimedia.org/wiki/Image:Stylised_Lithium_Atom.png. Creative Commons.
http://en.wikipedia.org/wiki/Image:Water_molecule.svg. Creative Commons.
http://en.wikipedia.org/wiki/Image:AminoAcidball.svg. Creative Commons.
http://en.wikipedia.org/wiki/Image:Water_drops_on_spider_web.jpg. Public Domain.
http://en.wikipedia.org/wiki/Image:2-amino-acidsb.png. Public Domain.
http://en.wikipedia.org/wiki/Image:
Water-elpot-transparent-3D-balls.png. Public Domain.
http://en.wikipedia.org/wiki/Image:Protein-primary-structure.png. Public Domain.
http://en.wikipedia.org/wiki/Image:Rasyslami.jpg. Creative Commons.
http://commons.wikimedia.org/wiki/Image:DNA_ORF.gif. Public Domain.
http://commons.wikimedia.org/wiki/File:Genetic_code.svg. CC-BY-SA.
www.ck12.org 144
Identify the scientists that first observed cells.
Outline the importance of microscopes in the discovery of cells.
Summarize what the cell theory proposes.
Identify the limitations on cell size.
Identify the three parts common to all cells.
Compare prokaryotic and eukaryotic cells.
Knowing the make up of cells and how cells work is necessary to all of the biological sciences. Learning about the similarities and differences between cell types is particularly important to the fields of cell biology and molecular biology. The importance of the similarities and differences between cell types is a unifying theme in biology. They allow the principles learned from studying one cell type to be applied when learning about other cell types. For example, learning about how single-celled animals or bacteria work can help us understand more about how human cells work. Research in cell biology is closely linked to genetics, biochemistry, molecular biology, and developmental biology.
A cell is the smallest unit that can carry out the processes of life. It is the basic unit of all living things, and all organisms are made up of one or more cells. In addition to having
the same basic structure, all cells carry out similar life processes. These include transport of materials, obtaining and using energy, waste disposal, replication, and responding to their environment.
If you look at living organisms under a microscope you will see they are made up of cells. The word cell was first used by Robert Hooke, a British biologist and early microscopist. Hooke looked at thin slices of cork under a microscope. The structure he saw looked like a honeycomb as it was made up of many tiny units. Hooke’s drawing is shown in Figure 3.1. In 1665 Hooke published his book Micrographia, in which he wrote:
... I could exceedingly plainly perceive it to be all perforated and porous, much like a Honey-comb, but that the pores of it were not regular.... these pores, or cells, ... were indeed the first microscopical pores I ever saw, and perhaps, that were ever seen, for I had not met with any Writer or Person, that had made any mention of them before this...
Figure 3.1: Drawing of the structure of cork from Micrographia as it appeared under the microscope to Robert Hooke. The first scientific use of the word cell appears in this book. (3)
During the 1670s, the Dutch tradesman Antony van Leeuwenhoek, shown in Figure 3.2, used microscopes to observe many microbes and body cells. Leeuwenhoek developed an interest in microscopy and ground his own lenses to make simple microscopes. Compound microscopes, which are microscopes that use more than one lens, had been invented around 1595. Several people, including Robert Hooke, had built compound microscopes and were making important discoveries with them during Leeuwenhoek’s time. These compound mi- croscopes were very similar to the microscopes in use today. However, Leeuwenhoek was so good at making lenses that his simple microscopes were able to magnify much more clearly
www.ck12.org 146
than the compound microscopes of his day. His microscope’s increased ability to magnify over 200 times is comparable to a modern compound light microscope.
Leeuwenhoek was also very curious, and he took great care in writing detailed reports of what he saw under his microscope. He was the first person to report observations of many micro- scopic organisms. Some of his discoveries included tiny animals such as ciliates, foraminifera, roundworms, and rotifers, shown in Figure 3.3. He discovered blood cells and was the first person to see living sperm cells. In 1683, Leeuwenhoek wrote to the Royal Society of London about his observations on the plaque between his own teeth, ”a little white matter, which is as thick as if ’twere batter.” He called the creatures he saw in the plaque animacules, or tiny animals. This report was among the first observations on living bacteria ever recorded.


Figure 3.3: Rotifers, similar to the type that Leeuwenhoek saw under his microscope. (5)
Hooke’s and Leeuwenhoek’s studies and observations filled people with wonder because their studies were of life forms that were everywhere, but too small to see with the naked eye. Just think how amazed you would be if you were to read about the first accounts of a newly discovered microorganism from the moon or Mars. Your first thought might be ”Things can live there?!” which was probably the first thought of the people who read Hooke’s and Leeuwenhoek’s accounts. The microscope literally opened up an amazing new dimension in the natural sciences, and became a critical tool in the progress of biology.
Magnifying glasses had been in use since the 1300s, but the use of lenses to see very tiny objects was a slowly-developing technology. The magnification power of early microscopes was very limited by the glass quality used in the lenses and the amount of light reflected off the object. These early light microscopes had poor resolution and a magnification power of about 10 times. Compare this to the over 200 times magnification that Leeuwenhoek was able to achieve by carefully grinding his own lenses. However, in time the quality of microscopes was much improved with better lighting and resolution. It was through the use of light microscopes that the first discoveries about the cell and the cell theory (1839) were developed.
However, by the end of the 19th century, light microscopes had begun to hit resolution limits. Resolution is a measure of the clarity of an image; it is the minimum distance that two points can be separated by and still be distinguished as two separate points. Because light beams have a physical size, it is difficult to see an object that is about the same size as the wavelength of light. Objects smaller than about 0.2 micrometers appear fuzzy, and objects below that size just cannot be seen. Light microscopes were still useful, but most of the organelles and tiny cell structures discussed in later lessons were invisible to the light microscope.
In the 1950s, a new system was developed that could use a beam of electrons to resolve very tiny dimensions at the molecular level. Electron microscopes, one of which is shown in Figure 3.4, have been used to produce images of molecules and atoms. They have been used to visualize the tiny sub-cellular structures that were invisible to light microscopes. Many of the discoveries made about the cell since the 1950s have been made with electron microscopes.
Later, biologists found cells everywhere. Biologists in the early part of the 19th century suggested that all living things were made of cells, but the role of cells as the primary building block of life was not discovered until 1839 when two German scientists, Theodor Schwann, a zoologist, and Matthias Jakob Schleiden, a botanist, suggested that cells were the basic unit of all living things. Later, in 1858, the German doctor Rudolf Virchow observed
www.ck12.org 148
Figure 3.4: Left to right: (a) Hooke’s light microscope (b) Modern electron microscope. (17)
that cells divide to produce more cells. He proposed that all cells arise only from other cells. The collective observations of all three scientists form the cell theory. The modern cell theory states that:
All organisms are made up of one or more cells.
All the life functions of an organism occur within cells.
All cells come from preexisting cells.
As with any theory, the cell theory is based on observations that over many years upheld the basic conclusions of Schwann’s paper written in 1839. However, one of Schwann’s original conclusions stated that cells formed in a similar way to crystals. This observation, which refers to spontaneous generation of life, was discounted when Virchow proposed that all cells arise only from other cells. The cell theory has withstood intense examination of cells by modern powerful microscopes and other instruments. Scientists use new techniques and equipment to look into cells to discover additional explanations for how they work.
Different cells within a single organism can come in a variety of sizes and shapes. They may not be very big, but their shapes can be very different from each other. However, these cells
all have common abilities, such as getting and using food energy, responding to the external environment, and reproducing. A cell’s shape determines its function.
If cells have such an important job, why are they so small? And why are there no organisms with huge cells? The answers to these questions lie in a cell’s need for fast, easy food. The need to be able to pass nutrients and gases into and out of the cell sets a limit on how big cells can be. The larger a cell gets, the more difficult it is for nutrients and gases to move in and out of the cell.
As a cell grows, its volume increases more quickly than its surface area. If a cell was to get very large, the small surface area would not allow enough nutrients to enter the cell quickly enough for the cell’s needs. This idea is explained in Figure 3.5. However, large cells have a way of dealing with some size challenges. Big cells, such as some white blood cells, often grow more nuclei so that they can supply enough proteins and RNA for the cell’s needs. Large, metabolically active cells often have lots of folds in their cell surface membrane. These folds increase the surface area available for transport into or out of the cell. Such cell types are found lining your small intestine, where they absorb nutrients from your food through little folds called microvilli.
1 centimeter (cm) = 10 millimeters (mm) = 10-2 meters (m) 1 mm = 1000 micrometers (µm) = 10-3 m
1 µm = 1000 nanometers (nm) = 10-6 m
1 nm = 10-3 µm
Imagine cells as little cube blocks. A small cube cell is one unit in length. www.ck12.org 150
The total surface area of this cell is calculated by the equation:
height × width × number of sides × number of boxes 1 × 1 × 6 × 1 = 6
The volume of the cell is calculated:
height x width x length x number of boxes 1 × 1 × 1 × 1 = 1
The surface-area to volume ratio is:
area ÷ volume 6 ÷ 1=6
A larger cell that is 3 units in length would have a total surface area of 3 × 3 × 6 × 1 = 54
and a volume of:
3 × 3 × 3 × 1 = 27
The surface-area to volume ratio of the large cell is:
54÷ 27=2
Now, replace the three unit cell with enough one unit cells to equal the volume of the single three unit cell. This can be done with 27 one unit cells. Find the total surface area of the 27 cells:
1 × 1 × 6 × 27 = 162 units
The total volume of the block of 27 cells is:
1 × 1 × 1 × 27 = 27
The surface-area to volume ratio of the 27 cells is:
162 ÷ 27=6
An increased surface area to volume ratio means increased exposure to the environment. This means that nutrients and gases can move in and out of a small cell more easily than in and out of a larger cell.
The smallest prokaryotic cell currently known has a diameter of only 400 nm. Eukaryotic cells normally range between 1– 100 µm in diameter.
The cells you have learned about so far are tinier than the period at the end of this sentence, so they are normally measured on a very tiny scale. Most cells are between 1 and 100 µm in diameter. The mouse cells in Figure 3.6 are about 10 µm in diameter. One exception

however, is eggs. Eggs contain the largest known single cell, and the ostrich egg is the largest of them all. The ostrich egg in Figure 3.6 is over 10,000 times larger than the mouse cell.
The variety of cell shapes seen in prokaryotes and eukaryotes reflects the functions that each cell has. Each cell type has evolved a shape that best helps it survive and do its job. For example, the nerve cell in Figure 3.7 has long, thin extensions that reach out to other nerve cells. The extensions help the nerve cell pass chemical and electrical messages quickly through the body. The spikes on the pollen grain help it stick to a pollinating insect or
www.ck12.org 152
animal so that it can be transferred to and pollinate another flower. The long whip-like flagella (tails) of the algae Chlamydomonas help it swim in water.
There are many different types of cells, but all cells have a few things in common. These are:
a cell or plasma membrane
cytoplasm
ribosomes for protein synthesis
DNA (genetic information)
The cell membrane is the physical boundary between the inside of the cell (intracellular) and its outside environment (extracellular). It acts almost like the ”skin” of the cell. Cy- toplasm is the general term for all of the material inside the cell. Cytoplasm is made up of cytosol, a watery fluid that contains dissolved particles and organelles. Organelles are structures that carry out specific functions inside the cell. Ribosomes are the organelles on which proteins are made. Ribosomes are found throughout the cytosol of the cell. All cells also have DNA. DNA contains the genetic information needed for building structures such as proteins and RNA molecules in the cell.
There are two cell types: prokaryotes and eukaryotes. Prokaryotic cells are usually single- celled and smaller than eukaryotic cells. Eukaryotic cells are usually found in multicellular organisms, but there are some single-celled eukaryotes.
The bacterium in Figure 3.8 is a prokaryote. Prokaryotes are organisms that do not have a cell nucleus nor any organelles that are surrounded by a membrane. Some cell biol- ogists consider the term ”organelle” to describe membrane-bound structures only, whereas other cell biologists define organelles as discrete structures that have a specialized function. Prokaryotes have ribosomes, which are not surrounded by a membrane but do have a special- ized function, and could therefore be considered organelles. Most of the metabolic functions carried out by a prokaryote take place in the plasma membrane.
Most prokaryotes are unicellular and have a cell wall that adds structural support and acts as a barrier against outside forces. Some prokaryotes have an extra layer outside their cell wall called a capsule, which helps them stick to surfaces or to each other. Prokaryotic DNA
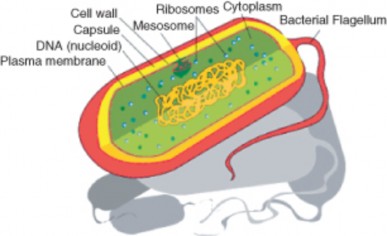
usually forms a circular molecule and is found in the cell’s cytoplasm along with ribosomes. Prokaryotic cells are very small; most are between 1–10 µm in diameter. They are found living in almost every environment on Earth. Biologists believe that prokaryotes were the first type of cells on Earth and that they are the most common organisms on Earth today.
A eukaryote is an organism whose cells are organized into complex structures by internal membranes and a cytoskeleton, as shown in Figure 3.13. The most characteristic membrane- bound structure of eukaryotes is the nucleus. This feature gives them their name, which comes from Greek and means ”true nucleus.” The nucleus is the membrane-enclosed or- ganelle that contains DNA. Eukaryotic DNA is organized in one or more linear molecules, called chromosomes. Some eukaryotes are single-celled, but many are multicellular.
In addition to having a plasma membrane, cytoplasm, a nucleus and ribosomes, eukaryotic cells also contain membrane-bound organelles. Each organelle in a eukaryote has a distinct function. Because of their complex level of organization, eukaryotic cells can carry out many more functions than prokaryotic cells. The main differences between prokaryotic and eukaryotic cells are shown in Figure 3.11 and listed in Table 1. Eukaryotic cells may or may not have a cell wall. Plant cells generally have cell walls, while animal cells do not.
Eukaryotic cells are about 10 times the size of a typical prokaryote; they range between 10 and 100 µm in diameter while prokaryotes range between 1 and 10 µm in diameter, as shown in Figure 3.10. Scientists believe that eukaryotes developed about 1.6 – 2.1 billion years ago. The earliest fossils of multicellular organisms that have been found are 1.2 billion years
www.ck12.org 154
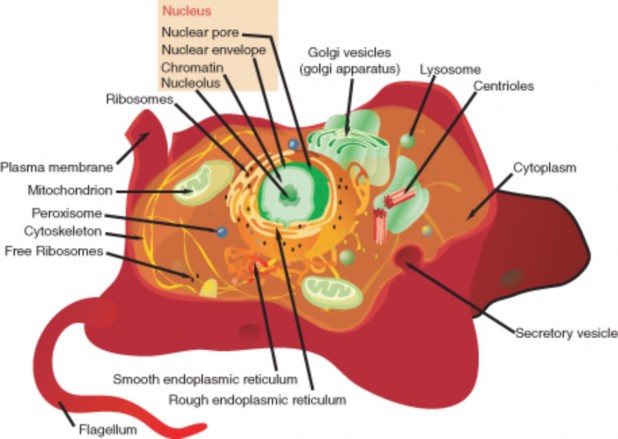
old.
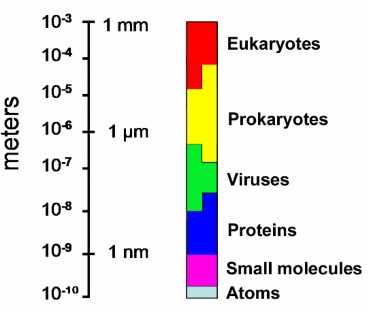
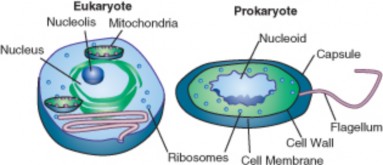
Table 3.1: Structural Differences Between Prokaryotic Cells and Eukaryotic Cells
Presence of | Prokaryote | Eukaryote | |
Plasma membrane Genetic material (DNA) Cytoplasm Ribosomes | yes yes yes yes | yes yes yes yes | |
156 |
Table 3.1: (continued)
![]()
Presence of Prokaryote Eukaryote
![]()
Nucleus no yes
Nucleolus no yes
Mitochondria no yes
Other membrane-bound or- ganelles
no yes
Cell wall yes some (not around animal cells)
Capsule yes no
Average diameter 0.4 to 10 µm 1 to 100 µm
![]()
Are viruses prokaryotic or eukaryotic? Neither. Viruses are not made up of cells, so they do not have a cell membrane or any cytoplasm, ribosomes, or other organelles. Viruses do not replicate by themselves, instead, they use their host cell to make more of themselves. So most virologists consider viruses non-living. But, they do evolve, which is a characteristic of living things.
A virus is a sub-microscopic particle that can infect living cells. Viruses are much smaller than prokaryotic organisms. In essence, a virus is simply a nucleic acid surrounded by a protein coat. Viruses will be discussed in more detail in the Prokaryotes and Viruses chapter.
Robert Hooke first saw and named cells. Antony van Leeuwenhoek was the first person to see living cells.
Before the development of microscopes, the existence of cellular life was unknown. The development of light microscopes and later electron microscopes helped scientists learn more about the cell. Most of the discoveries about cell structure since the 1950s have been made due to the use of electron microscopes.
The cell theory states that all living things are made of one or more cells, that cells are the basic unit of life, and that cells come only from other cells.
Cell size is limited by a cell’s surface area to volume ratio. A cell’s shape is determined by its function.
Parts common to all cells are the plasma membrane, the cytoplasm, ribosomes, and genetic material.
Prokaryotic cells lack a nucleus and other membrane-bound organelles.
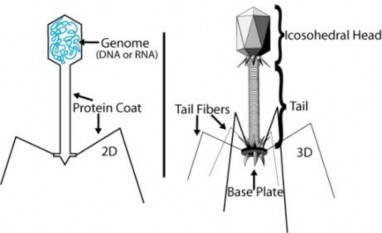
Describe the contributions of Hooke and Leeuwenhoek to cell biology.
What enabled Leeuwenhoek to observe things that nobody else had seen before?
What three things does the cell theory propose?
A cell has a volume of 64 units, and total surface area of 96 units. What is the cell’s surface area to volume ratio (surface area ÷ volume)?
What is the relationship between cell shape and function?
What are the three basic parts of a cell?
Compare prokaryotic and eukaryotic cells. Identify two differences between prokaryotic and eukaryotic cells.
Is the cell in this image prokaryotic or eukaryotic? Explain your answer.
Human Anatomy © 2003 Martini, Timmons, Tallitsch. Published by Prentice Hall, Inc.
http://www.brianjford.com/wav-mict.htm
www.ck12.org 158
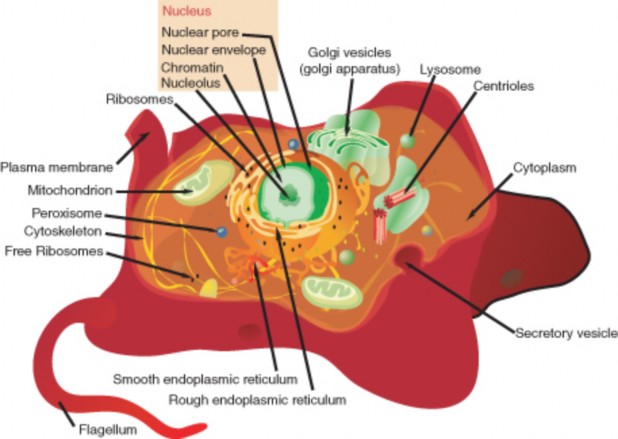
http://cellimages.ascb.org/cdm4/browse.php?CISOROOT=/p4041coll11
cell The smallest unit that can carry out the processes of life; the basic unit of all living things.
cell membrane The physical boundary between the inside of the cell (intracellular) and its outside environment (extracellular).
cytoplasm The general term for all of the material inside the cell, between the cell mem- brane and the nucleus.
cytosol A watery fluid that contains dissolved particles and organelles; makes up cyto- plasm.
DNA Deoxyribonucleic acid, the genetic material; contains the genetic information needed for building structures such as proteins.
eukaryote An organism whose cells are organized into complex structures by internal membranes and a cytoskeleton.
eukaryotic cells Typical of multi-celled organisms; have membrane bound organelles; usu- ally larger than prokaryotic cells.
nucleus The membrane bound organelle that contains DNA; found in eukaryotic cells.
organelle Structure that carries out specific functions inside the cell.
prokaryotic cells Typical of simple, single-celled organisms, such as bacteria; lack a nu- cleus and other membrane bound organelles.
resolution A measure of the clarity of an image; the minimum distance that two points can be separated by and still be distinguished as two separate points.
ribosomes The organelles on which proteins are made (synthesized). www.ck12.org 160
Next we focus on cell structures and their roles.
What do you think is the most important structure in a cell? Why?
How do you think cells stay intact? What keeps the insides of a cell separate from the outside of the cell?
Outline the structure of the plasma membrane.
Distinguish cytoplasm from cytosol.
Name three types of protein fibers that make up the cytoskeleton.
Distinguish between cilia and flagella.
Identify three structures that plant cells have but animal cells do not.
List three major organelles found only in eukaryotic cells and identify their roles.
Distinguish between a colonial organism and a multicellular organism.
Outline the relationship between cells, tissues, organs, and organ systems.
The invention of the microscope opened up a previously unknown world. Before the invention of the microscope, very little was known about what made up living things and non-living things, or where living things came from. During Hooke’s and Leeuwenhoek’s time, spon- taneous generation — the belief that living organisms grow directly from decaying organic substances — was the accepted explanation for the appearance of small organisms. For example, people accepted that mice spontaneously appeared in stored grain, and maggots formed in meat with no apparent external influence. Once cells were discovered, the search for answers to such questions as ”what are cells made of?” and ”what do they do?” became the focus of study.
Cells share the same needs: the need to get energy from their environment, the need to respond to their environment, and the need to reproduce. Cells must also be able to separate their relatively stable interior from the ever-changing external environment. They do this by coordinating many processes that are carried out in different parts of the cell. Structures that are common to many different cells indicate the common history shared by cell-based life.
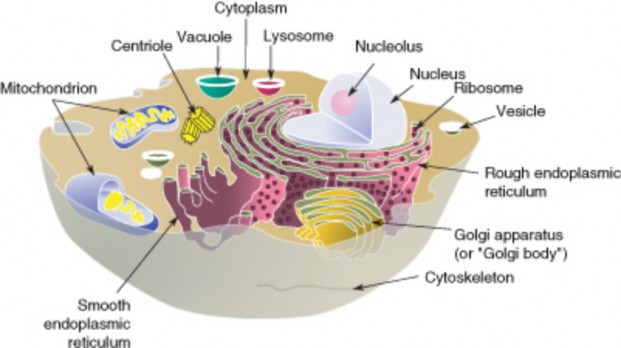
www.ck12.org 162
Examples of these common structures include the components of both the cell (or plasma) membrane and the cytoskeleton, and other structures shown in Figure 3.14.
The plasma membrane (also called the cell membrane) has many functions. For example, it separates the internal environment of the cell from the outside environment. It allows only certain molecules into and out of the cell. The ability to allow only certain molecules in or out of the cell is referred to as selective permeability or semipermeability. The plasma membrane also acts as the attachment point for both the intracellular cytoskeleton and, if present, the cell wall.
The plasma membrane is a lipid bilayer that is common to all living cells. A lipid bilayer is a double layer of closely-packed lipid molecules. The membranes of cell organelles are also lipid bilayers. The plasma membrane contains many different biological molecules, mostly lipids and proteins. These lipids and proteins are involved in many cellular processes.
The main type of lipid found in the plasma membrane is phospholipid. A phospholipid is made up of a polar, phosphorus-containing head, and two long fatty acid, non-polar ”tails.” That is, the head of the molecule is hydrophilic (water-loving), and the tail is hydrophobic (water-fearing). Cytosol and extracellular fluid are made up of mostly water. In this watery environment, the water loving heads point out towards the water, and the water fearing tails point inwards, and push the water out. The resulting double layer is called a phospholipid bilayer. A phospholipid bilayer is made up of two layers of phospholipids, in which hydrophobic fatty acids are in the middle of the plasma membrane, and the hydrophilic heads are on the outside. An example of a simple phospholipid bilayer is illustrated in Figure 3.15.
Plasma membranes of eukaryotes contain many proteins, as well as other lipids called sterols. The proteins have various functions, such as channels that allow certain molecules into the cell and receptors that bind to signal molecules. In Figure 3.15, the smaller (green) molecules shown between the phospholipids are cholesterol molecules. Cholesterol helps keep the plasma membrane firm and stable over a wide range of temperatures. At least ten different types of lipids are commonly found in plasma membranes. Each type of cell or organelle will have a different percentage of each lipid, protein and carbohydrate.
Plasma membranes also contain certain types of proteins. A membrane protein is a protein molecule that is attached to, or associated with the membrane of a cell or an organelle.
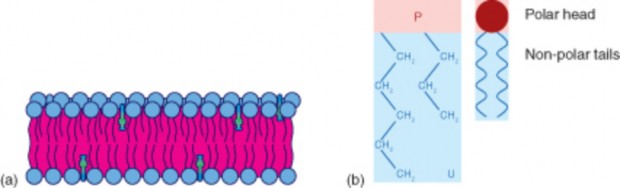
Figure 3.15: a) The hydrophobic fatty acids point towards the middle of the plasma mem- brane (pink), and the hydrophilic heads (blue) point outwards. The membrane is stabilized by cholesterol molecules (green). b) This self-organization of phospholipids results in a semipermeable membrane which allows only certain molecules in or out of the cell. (12)
Membrane proteins can be put into two groups based on how the protein is associated with the membrane.
Integral membrane proteins are permanently embedded within the plasma membrane. They have a range of important functions. Such functions include channeling or transporting molecules across the membrane. Other integral proteins act as cell receptors. Integral membrane proteins can be classified according to their relationship with the bilayer:
Transmembrane proteins span the entire plasma membrane. Transmembrane proteins are found in all types of biological membranes.
Integral monotopic proteins are permanently attached to the membrane from only one side.
Some integral membrane proteins are responsible for cell adhesion (sticking of a cell to another cell or surface). On the outside of cell membranes and attached to some of the proteins are carbohydrate chains that act as labels that identify the cell type. Shown in Figure 3.16 are two different types of membrane proteins and associated molecules.
Peripheral membrane proteins are proteins that are only temporarily associated with the membrane. They can be easily removed, which allows them to be involved in cell signaling. Peripheral proteins can also be attached to integral membrane proteins, or they can stick into a small portion of the lipid bilayer by themselves. Peripheral membrane proteins are often associated with ion channels and transmembrane receptors. Most peripheral membrane proteins are hydrophilic.
www.ck12.org 164
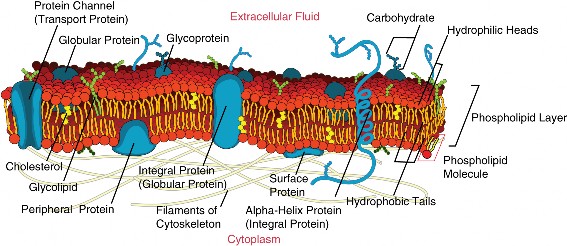
In 1972 S.J. Singer and G.L. Nicolson proposed the now widely accepted Fluid Mosaic Model of the structure of cell membranes. The model proposes that integral membrane proteins are embedded in the phospholipid bilayer, as seen in Figure 3.16. Some of these proteins extend all the way through the bilayer, and some only partially across it. These membrane proteins act as transport proteins and receptors proteins.
Their model also proposed that the membrane behaves like a fluid, rather than a solid. The proteins and lipids of the membrane move around the membrane, much like buoys in water. Such movement causes a constant change in the ”mosaic pattern” of the plasma membrane.
The gel-like material within the cell that holds the organelles is called cytoplasm. The cytoplasm plays an important role in a cell, serving as a ”jelly” in which organelles are suspended and held together by a fatty membrane. The cytosol, which is the watery substance that does not contain organelles, is made up of 80% to 90% water.
The cytosol plays a mechanical role by exerting pressure against the cell’s plasma membrane which helps keep the shape of the cell. Cytosol also acts as the site of biochemical reactions such as anaerobic glycolysis and protein synthesis. In prokaryotes all chemical reactions take place in the cytosol.
The cytoskeleton is a cellular ”scaffolding” or ”skeleton” that crisscrosses the cytoplasm. All eukaryotic cells have a cytoskeleton, and recent research has shown that prokaryotic cells also have a cytoskeleton. The eukaryotic cytoskeleton is made up of a network of long, thin protein fibers and has many functions. It helps to maintain cell shape. It holds organelles in place, and for some cells, it enables cell movement. The cytoskeleton also plays important roles in both the intracellular movement of substances and in cell division. Certain proteins act like a path that vesicles and organelles move along within the cell. The threadlike proteins that make up the cytoskeleton continually rebuild to adapt to the cell’s constantly changing needs. Three main kinds of cytoskeleton fibers are microtubules, intermediate filaments, and microfilaments.
Microtubules, shown in Figure (a), are hollow cylinders and are the thickest of the cytoskeleton structures. They are most commonly made of filaments which are polymers of alpha and beta tubulin, and radiate outwards from an area near the nucleus called the centrosome. Tubulin is a protein that is composed of hollow cylinders which are made of two protein chains that are twisted around each other. Microtubules help keep cell shape. They hold organelles in place and allow them to move around the cell, and they form the mitotic spindle during cell division. Microtubules also make up parts of cilia and flagella, the organelles that help a cell to move.
Microfilaments, shown in Figure (b), are made of two thin actin chains that are twisted around one another. Microfilaments are mostly concentrated just beneath the cell membrane where they support the cell and help keep the cell’s shape. Mi- crofilaments form cytoplasmatic extentions such as pseudopodia and microvilli which allows certain cells to move. The actin of the microfilaments interacts with the protein myosin to cause contraction in muscle cells. Microfilaments are found in almost every cell, and are numerous in muscle cells and in cells that move by changing shape such as phagocytes (white blood cells that search the body for bacteria and other invaders).
Intermediate filament, shown in Figure (c), make-up differs from one cell type to another. Intermediate filaments organize the inside structure of the cell by holding organelles and providing strength. They are also structural components of the nuclear envelope. Intermediate filaments made of the protein keratin are found in skin, hair, and nails cells.
www.ck12.org 166
(a)The eukaryotic cytoskeleton. Microfilaments are shown in red, microtubules in green, and the nuclei are in blue. By linking regions of the cell together, the cytoskeleton helps support the shape of the cell. (b) Microscopy of keratin filaments (intermediate filaments) inside cells. (c) Microtubules in a methanol-fixated cell, visualized with anti-beta-tubuline antibodies.
Table 3.2: Cytoskeleton Structure
![]()
Microtubules Intermediate Fila- ments
Microfilaments
![]()
Fiber Diameter About 25 nm 8 to 11 nm Around 7 nm
Tubulin, with two subunits, alpha and beta tubulin
One of different types of proteins such as lamin, vi- mentin, and keratin
Actin
Shape Hollow cylinders made of two pro- tein chains twisted around each other
Main Functions Organelle and vesi-
cle movement; form mitotic spindles dur- ing cell reproduc- tion; cell motility (in cilia and flagella)

Protein fiber coils twisted into each other
Organize cell shape; positions organelles in cytoplasm struc- tural support of the nuclear envelope and sarcomeres; involved in cell-to-cell and cell-to-matrix junc- tions
Two actin chains twisted around one another
Keep cellular shape; allows movement of certain cells by forming cytoplas- matic extensions or contraction of actin fibers; involved in some cell-to-cell or cell-to-matrix junctions
Molecular structure
of microtubules. Keratin intermedi-
ate filaments in skin cells (stained red).
Actin cytoskeleton of mouse embryo cells.
![]()
Flagella (flagellum, singular) are long, thin structures that stick out from the cell membrane. Both eukaryotic and prokaryotic cells can have flagella. Flagella help single-celled organisms move or swim towards food. The flagella of eukaryotic cells are normally used for movement too, such as in the movement of sperm cells. The flagella of either group are very different from each other. Prokaryotic flagella, shown below, are spiral-shaped and stiff. They spin around in a fixed base much like a screw does, which moves the cell in a tumbling fashion. Eukaryotic flagella are made of microtubules and bend and flex like a whip.
Bacterial flagella spin about in place, which causes the bacterial cell to ”tumble.”
Cilia (cilium, singular) are made up of extensions of the cell membrane that contain micro- tubules. Although both are used for movement, cilia are much shorter than flagella. Cilia cover the surface of some single-celled organisms, such as paramecium. Their cilia beat together to move the little animals through the water. In multicellular animals, including humans, cilia are usually found in large numbers on a single surface of cells. Multicellular animals’ cilia usually move materials inside the body. For example, the mucociliary escala- tor of the respiratory system is made up of mucus-secreting cells that line the trachea and bronchi. Ciliated cells, shown in Figure 3.17, move mucus away from the lungs. Spores, bacteria, and debris are caught in the mucus which is moved to the esophagus by the ciliated cells, where it is swallowed.
The nucleus is a membrane-enclosed organelle found in most eukaryotic cells. The nucleus is the largest organelle in the cell and contains most of the cell’s genetic information (mitochon- dria also contain DNA, called mitochondrial DNA, but it makes up just a small percentage of the cell’s overall DNA content). The genetic information, which contains the information for the structure and function of the organism, is found encoded in DNA in the form of genes. A gene is a short segment of DNA that contains information to encode an RNA molecule or a protein strand. DNA in the nucleus is organized in long linear strands that are attached to different proteins. These proteins help the DNA to coil up for better storage
www.ck12.org 168
in the nucleus. Think how a string gets tightly coiled up if you twist one end while holding the other end. These long strands of coiled-up DNA and proteins are called chromosomes. Each chromosome contains many genes. The function of the nucleus is to maintain the in- tegrity of these genes and to control the activities of the cell by regulating gene expression. Gene expression is the process by which the information in a gene is ”decoded” by various cell molecules to produce a functional gene product, such as a protein molecule or an RNA molecule.
The degree of DNA coiling determines whether the chromosome strands are short and thick or long and thin. Between cell divisions, the DNA in chromosomes is more loosely coiled and forms long thin strands called chromatin. Before the cell divides, the chromatin coil up more tightly and form chromosomes. Only chromosomes stain clearly enough to be seen under a microscope. The word chromosome comes from the Greek word chroma, (color) and soma, (body) due to its ability to be stained strongly by dyes.
The nuclear envelope is a double membrane of the nucleus that encloses the genetic ma- terial. It separates the contents of the nucleus from the cytoplasm. The nuclear envelope is made of two lipid bilayers, an inner membrane and an outer membrane. The outer mem- brane is continuous with the rough endoplasmic reticulum. Many tiny holes called nuclear pores are found in the nuclear envelope. These nuclear pores help to regulate the exchange of materials (such as RNA and proteins) between the nucleus and the cytoplasm.
The nucleus of many cells also contains an organelle called a nucleolus, shown in Figure
The nucleolus is mainly involved in the assembly of ribosomes. Ribosomes are organelles made of protein and ribosomal RNA (rRNA), and they build cellular proteins in the cytoplasm. The function of the rRNA is to provide a way of decoding the genetic messages within another type of RNA called mRNA, into amino acids. After being made in the nucleolus, ribosomes are exported to the cytoplasm where they direct protein synthesis.
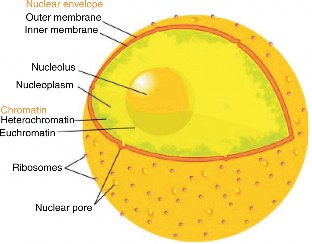
Centrioles are rod-like structures made of short microtubules. Nine groups of three mi- crotubules make up each centriole. Two perpendicularly placed centrioles make up the cen- trosome. Centrioles are very important in cellular division, where they arrange the mitotic spindles that pull the chromosome apart during mitosis.
www.ck12.org 170
A mitochondrion (mitochondria, plural), is a membrane-enclosed organelle that is found in most eukaryotic cells. Mitochondria are called the ”power plants” of the cell because they use energy from organic compounds to make ATP. ATP is the cell’s energy source that is used for such things such as movement and cell division. Some ATP is made in the cytosol of the cell, but most of it is made inside mitochondria. The number of mitochondria in a cell depends on the cell’s energy needs. For example, active human muscle cells may have thousands of mitochondria, while less active red blood cells do not have any.
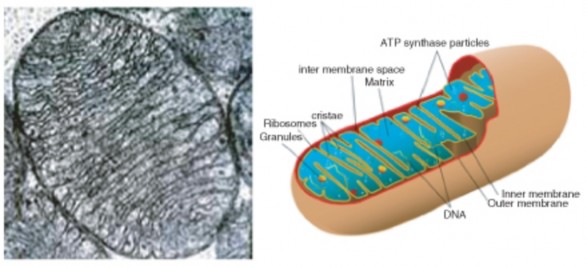
Figure 3.19: (a): Electron micrograph of a single mitochondrion within which you can see many cristae. Mitochondria range from 1 to 10 m in size. (b): This model of a mitochondrian shows the organized arrangement of the inner and outer membranes, the protein matrix, and the folded inner mitochondrial membranes. (19)
As Figure 3.19 (a) and (b) shows, a mitochondrion has two phospholipids membranes. The smooth outer membrane separates the mitochondrion from the cytosol. The inner membrane has many folds, called cristae. The fluid-filled inside of the mitochondrian, called matrix, is where most of the cell’s ATP is made.
Although most of a cell’s DNA is contained in the cell nucleus, mitochondria have their own DNA. Mitochandria are able to reproduce asexually and scientists think that they are descended from prokaryotes. According to the endosymbiotic theory, mitochondria were once free-living prokaryotes that infected ancient eukaryotic cells. The invading prokaryotes were protected inside the eukaryotic host cell, and in turn the prokaryote supplied extra ATP to its host.
The endoplasmic reticulum (ER) (plural, reticuli) is a network of phospholipid mem- branes that form hollow tubes, flattened sheets, and round sacs. These flattened, hollow folds and sacs are called cisternae. The ER has two major functions:
Transport: Molecules, such as proteins, can move from place to place inside the ER, much like on an intracellular highway.
Synthesis: Ribosomes that are attached to ER, similar to unattached ribosomes, make proteins. Lipids are also produced in the ER.
There are two types of endoplasmic reticulum, rough endoplasmic reticulum (RER) and smooth endoplasmic reticulum (SER).
Rough endoplasmic reticulum is studded with ribosomes which gives it a ”rough” appearance. These ribosomes make proteins that are then transported from the ER in small sacs called transport vesicles. The transport vesicles pinch off the ends of the ER. The rough endoplasmic reticulum works with the Golgi apparatus to move new proteins to their proper destinations in the cell. The membrane of the RER is continuous with the outer layer of the nuclear envelope.
Smooth endoplasmic reticulum does not have any ribosomes attached to it, and so it has a smooth appearance. SER has many different functions some of which are: lipid synthesis, calcium ion storage, and drug detoxification. Smooth endoplasmic reticulum is found in both animal and plant cells and it serves different functions in each. The SER is made up of tubules and vesicles that branch out to form a network. In some cells there are dilated areas like the sacs of RER. Smooth endoplasmic reticulum and RER form an interconnected network.
Ribosomes are small organelles and are the site of protein synthesis (or assembly). They are made of ribosomal protein and ribosomal RNA. Each ribosome has two parts, a large and a small subunit, as shown in Figure 3.21. The subunits are attached to each other. Ribosomes can be found alone or in groups within the cytoplasm. Some ribosomes are attached to the endoplasmic reticulum (as shown in Figure 3.20), and others are attached to the nuclear envelope.
Ribozymes are RNA molecules that catalyzes chemical reactions, such as translation. Trans- lation is the process of ordering the amino acids in the assembly of a protein, and more will be discussed on translation in a later chapter. Briefly, the ribosomes interact with other RNA molecules to make chains of amino acids called polypeptide chains, due to the peptide
www.ck12.org 172
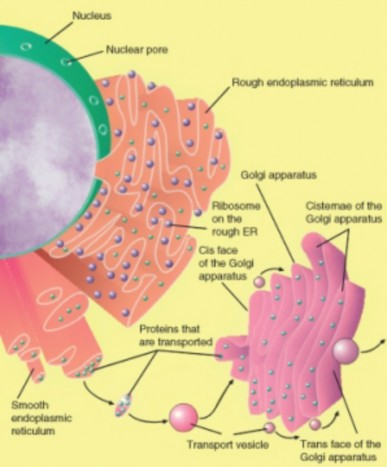
bond that forms between individual amino acids. Polypeptide chains are built from the genetic instructions held within a messenger RNA molecule. Polypeptide chains that are made on the rough ER are inserted directly into the ER and then are transported to their various cellular destinations. Ribosomes on the rough ER usually produce proteins that are destined for the cell membrane.
The Golgi apparatus is a large organelle that is usually made up of five to eight cup-shaped, membrane-covered discs called cisternae, as shown in Figure 3.20. The cisternae look a bit like a stack of deflated balloons. The Golgi apparatus modifies, sorts, and packages different substances for secretion out of the cell, or for use within the cell. The Golgi apparatus is found close to the nucleus of the cell where it modifies proteins that have been delivered in transport vesicles from the RER. It is also involved in the transport of lipids around the cell. Pieces of the Golgi membrane pinch off to form vesicles that transport molecules around the cell. The Golgi apparatus can be thought of as similar to a post office; it packages and labels ”items” and then sends them to different parts of the cell. Both plant and animal cells have a Golgi apparatus. Plant cells can have up to several hundred Golgi stacks scattered throughout the cytoplasm. In plants, the Golgi apparatus contains enzymes that synthesize some of the cell wall polysaccharides.
www.ck12.org 174
A vesicle is a small, spherical compartment that is separated from the cytosol by at least one lipid bilayer. Many vesicles are made in the Golgi apparatus and the endoplasmic reticulum, or are made from parts of the cell membrane. Vesicles from the Golgi apparatus can be seen in Figure 3.20. Because it is separated from the cytosol, the space inside the vesicle can be made to be chemically different from the cytosol. Vesicles are basic tools of the cell for organizing metabolism, transport, and storage of molecules. Vesicles are also used as chemical reaction chambers. They can be classified by their contents and function.
Transport vesicles are able to move molecules between locations inside the cell. For example, transport vesicles move proteins from the rough endoplasmic reticulum to the Golgi apparatus.
Lysosomes are vesicles that are formed by the Golgi apparatus. They contain powerful enzymes that could break down (digest) the cell. Lysosomes break down harmful cell products, waste materials, and cellular debris and then force them out of the cell. They also digest invading organisms such as bacteria. Lysosomes also break down cells that are ready to die, a process called autolysis.
Peroxisomes are vesicles that use oxygen to break down toxic substances in the cell. Unlike lysosomes, which are formed by the Golgi apparatus, peroxisomes self replicate by growing bigger and then dividing. They are common in liver and kidney cells that break down harmful substances. Peroxisomes are named for the hydrogen peroxide (H2O2) that is produced when they break down organic compounds. Hydrogen peroxide is toxic, and in turn is broken down into water (H2O) and oxygen (O2) molecules.
Vacuoles are membrane-bound organelles that can have secretory, excretory, and storage functions. Many organisms will use vacuoles as storage areas and some plant cells have very large vacuoles. Vesicles are much smaller than vacuoles and function in transporting materials both within and to the outside of the cell.
Most of the organelles that have been discussed are common to both animal and plant cells. However, plant cells also have features that animal cells do not have; they have a cell wall, a large central vacuole, and plastids such as chloroplasts.
Plants have very different lifestyles from animals, and these differences are apparent when you examine the structure of the plant cell. Plants make their own food in a process called photosynthesis. They take in carbon dioxide (CO2) and water (H2O) and convert them into sugars. The features unique to plant cells can be seen in Figure 3.22.
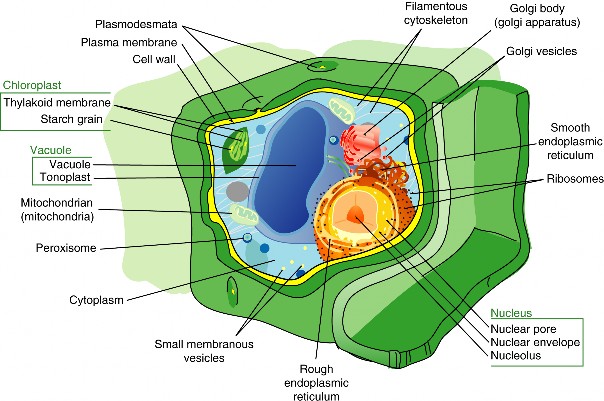
www.ck12.org 176
A cell wall is a rigid layer that is found outside the cell membrane and surrounds the cell. The cell wall contains not only cellulose and protein, but other polysaccharides as well. In fact, two other classes of polysaccharides, hemicelluloses and pectic polysaccharides, can comprise 30% of the dry mass of the cell wall. The cell wall provides structural support and protection. Pores in the cell wall allow water and nutrients to move into and out of the cell. The cell wall also prevents the plant cell from bursting when water enters the cell.
Microtubules guide the formation of the plant cell wall. Cellulose is laid down by enzymes to form the primary cell wall. Some plants also have a secondary cell wall. The secondary wall contains a lignin, a secondary cell component in plant cells that have completed cell growth/expansion.
Most mature plant cells have a central vacuole that occupies more than 30% of the cell’s volume, but can also occupy as much as 90% of the volume of certain cells. The central vacuole is surrounded by a membrane called the tonoplast. The central vacuole has many functions. Aside from storage, the main role of the vacuole is to maintain turgor pressure against the cell wall. Proteins found in the tonoplast control the flow of water into and out of the vacuole. The central vacuole also stores the pigments that color flowers.
The central vacuole contains large amounts of a liquid called cell sap, which differs in com- position to the cell cytosol. Cell sap is a mixture of water, enzymes, ions, salts, and other substances. Cell sap may also contain toxic byproducts that have been removed from the cytosol. Toxins in the vacuole may help to protect some plants from being eaten.
Plant plastids are a group of closely related membrane-bound organelles that carry out many functions. They are responsible for photosynthesis, for storage of products such as starch, and for the synthesis of many types of molecules that are needed as cellular building blocks. Plastids have the ability to change their function between these and other forms. Plastids contain their own DNA and some ribosomes, and scientists think that plastids are descended from photosynthetic bacteria that allowed the first eukaryotes to make oxygen. The main types of plastids and their functions are:
Chloroplasts are the organelle of photosynthesis. They capture light energy from the sun and use it with water and carbon dioxide to make food (sugar) for the plant. The arrangement of chloroplasts in a plant’s cells can be seen in Figure 3.23.
Chromoplasts make and store pigments that give petals and fruit their orange and yellow colors.
Leucoplasts do not contain pigments and are located in roots and non-photosynthetic tissues of plants. They may become specialized for bulk storage of starch, lipid, or protein. However, in many cells, leucoplasts do not have a major storage function; instead they make molecules such as fatty acids and many amino acids.
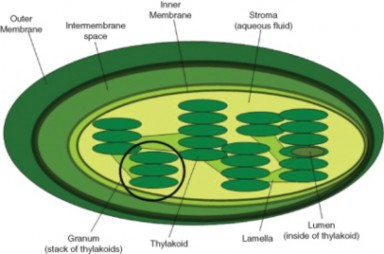
Figure 3.24: The internal structure of a chloroplast, with a granal stack of thylakoids circled. (15)
Chloroplasts capture light energy from the sun and use it with water and carbon dioxide to produce sugars for food. Chloroplasts look like flat discs that are usually 2 to 10 micrometers in diameter and 1 micrometer thick. A model of a chloroplast is shown in Figure 3.24. The chloroplast is enclosed by an inner and an outer phospholipid membrane. Between these two layers is the intermembrane space. The fluid within the chloroplast is called the stroma, and it contains one or more molecules of small circular DNA. The stroma also has
www.ck12.org 178
ribosomes. Within the stroma are stacks of thylakoids, the sub-organelles which are the site of photosynthesis. The thylakoids are arranged in stacks called grana (singular: granum). A thylakoid has a flattened disk shape. Inside it is an empty area called the thylakoid space or lumen. Photosynthesis takes place on the thylakoid membrane.
Within the thylakoid membrane is the complex of proteins and light-absorbing pigments, such as chlorophyll and carotenoids. This complex allows capture of light energy from many wavelengths because chlorophyll and carotenoids both absorb different wavelengths of light. You will learn more about how chloroplasts convert light energy into chemical energy in the Photosynthesis chapter.
Biological organization exists at all levels in organisms. It can be seen at the smallest level, in the molecules that made up such things as DNA and proteins, to the largest level, in an organism such as a blue whale, the largest mammal on Earth. Similarly, single celled prokaryotes and eukaryotes show order in the way their cells are arranged. Single-celled organisms such as an amoeba are free-floating and independent-living. Their single-celled ”bodies” are able to carry out all the processes of life such as metabolism and respiration without help from other cells. Some single-celled organisms such as bacteria can group together and form a biofilm. A biofilm is a large grouping of many bacteria that sticks to a surface and makes a protective coating over itself. Biofilms can show similarities to multicellular organisms. Division of labor is the process in which one group of cells does one job (such as making the ”glue” that sticks the biofilm to the surface) while another group of cells does another job (such as taking in nutrients). Multicellular organisms carry out their life processes through division of labor and they have specialized cells that do specific jobs. However, biofilms are not considered a multicellular organism and are instead called colonial organisms. The difference between a multicellular organism and a colonial organism is that individual organisms from a colony or biofilm can, if separated, survive on their own, while cells from a multicellular organism (e.g., liver cells) cannot.
Colonial organisms were probably one of the first evolutionary steps towards multicellular or- ganisms. Algae of the genus Volvox are an example of the border between colonial organisms and multicellular organisms.
Each Volvox, shown in Figure 3.25, is a colonial organism. It is made up of between 1000 to 3000 photosynthetic algae that are grouped together into a hollow sphere. The sphere has a distinct front and back end. The cells have eyespots, which are more developed in the cells near the front. This enables the colony to swim towards light.
Figure 3.25: Colonial algae of the genus Volvox. (27)
The oldest known multicellular organism is a red algae Bangiomorpha pubescens, fossils of which were found in 1.2 billion year old rock. However, the first organisms were single celled. How multicellular organisms developed is the subject of much debate.
Scientists think that multicellularity arose from cooperation between many organisms of the same species. The Colonial Theory proposes that this cooperation led to the development of a multicellular organism. Many examples of cooperation between organisms in nature have been observed. For example, a certain species of amoeba (a single-celled animal) groups together during times of food shortage and forms a colony that moves as one to a new location. Some of these amoebas then become slightly differentiated from each other. Volvox, shown in Figure 3.25, is another example of a colonial organism. Most scientists accept that the Colonial theory explains how multicellular organisms evolved.
Multicellular organisms are organisms that are made up of more than one type of cell and have specialized cells that are grouped together to carry out specialized functions. Most life that you can see without a microscope is multicellular. As discussed earlier, the cells of a multicellular organism would not survive as independent cells. The body of a multicellular organism, such as a tree or a cat, exhibits organization at several levels: tissues, organs, and organ systems. Similar cells are grouped into tissues, groups of tissues make up organs, and organs with a similar function are grouped into an organ system.
The simplest living multicellular organisms, sponges, are made of many specialized types of cells that work together for a common goal. Such cell types include digestive cells, tubular
www.ck12.org 180
pore cells; and epidermal cells. Though the different cell types create a large organized, multicellular structure—the visible sponge—they are not organized into true interconnected tissues. If a sponge is broken up by passing it through a sieve, the sponge will reform on the other side. However, if the sponge’s cells are separated from each other, the individual cell types cannot survive alone. Simpler colonial organisms, such as members of the genus Volvox, as shown in Figure 3.25, differ in that their individual cells are free-living and can survive on their own if separated from the colony.
A tissue is a group of connected cells that have a similar function within an organism. More complex organisms such as jellyfish, coral, and sea anemones have a tissue level of organization. For example, jellyfish have tissues that have separate protective, digestive, and sensory functions.
Even more complex organisms, such as the roundworm shown in Figure 3.26, while also having differentiated cells and tissues, have an organ level of development. An organ is a group of tissues that has a specific function or group of functions. Organs can be as primitive as the brain of a flatworm (a group of nerve cells), as large as the stem of a sequoia (up to 90 meters, or 300 feet, in height), or as complex as a human liver.
The most complex organisms (such as mammals, trees, and flowers) have organ systems. An organ system is a group of organs that act together to carry out complex related functions, with each organ focusing on a part of the task. An example is the human digestive system in which the mouth ingests food, the stomach crushes and liquifies it, the pancreas and gall
bladder make and release digestive enzymes, and the intestines absorb nutrients into the blood.
The plasma membrane is a selectively permeable lipid bilayer that contains mostly lipids and proteins. These lipids and proteins are involved in many cellular processes.
The gel-like material within the cell that holds the organelles is called cytoplasm. The cytosol, which is the watery substance that does not contain organelles, is made up of 80% to 90% water.
The cytoskeleton has many functions. It helps to maintain cell shape, it holds organelles in place, and for some cells, it enables cell movement. The cytoskeleton also plays important roles in both the intracellular movement of substances and in cell division. Three main kinds of cytoskeleton fibers are microtubules, intermediate filaments, and microfilaments.
Cilia are extensions of the cell membrane that contain microtubules. Although both are used for movement, cilia are much shorter than flagella. Cilia cover the surface of some single-celled animals, such as paramecium, but cover only one side of cells in some multicellular organisms.
There are three features that plant cells have that animal cells do not have: a cell wall, a large central vacuole, and plastids.
Mitochondria use energy from organic compounds to make ATP.
Ribosomes are exported from the nucleolus, where they are made, to the cytoplasm.
The Golgi apparatus is a large organelle that is usually made up of five to eight cup- shaped, membrane-covered discs called cisternae. It modifies, sorts, and packages different substances for secretion out of the cell, or for use within the cell.
Individual organisms from a colonial organism or biofilm can, if separated, survive on their own, while cells from a multicellular organism (e.g., liver cells) cannot.
A tissue is a group of connected cells that have a similar function within an organism. An organ is a group of tissues that has a specific function or group of functions, and an organ system is a group of organs that act together to perform complex related functions, with each organ focusing on a part of the task.
The following web site is an interactive representation of a plant and animal cell, with their various organelles.
The following animation is a detailed example of the functions of the specific parts of the cell.
www.ck12.org 182
http://www.johnkyrk.com/er.html
The following site is a virtual cell where various organelles can be observed.
http://www.ibiblio.org/virtualcell/tour/cell/cell.htm
Department of Biological Sciences, Carnegie Mellon University
http://telstar.ote.cmu.edu/biology/
What are the main components of a plasma membrane?
What does the fluid mosaic model describe?
What is the difference between cytoplasm and cytosol?
What type of molecule is common to all three parts of the cytoskeleton?
Name the three main parts of the cytoskeleton.
What structures do plant cells have that animal cells do not have?
Identify two functions of plastids in plant cells.
What is the main difference between rough endoplasmic reticulum and smooth endo- plasmic reticulum?
List five organelles eukaryotes have that prokaryotes do not have.
What is a cell feature that distinguishes a colonial organism from a multicellular or- ganism?
What is the difference between a cell and a tissue?
Identify two functions of the nucleus.
Identify the reason why mitochondria are called ”power plants” of the cell.
If muscle cells become more active than they usually are, they will grow more mito- chondria. Explain why this happens.
N. J. Butterfield (2000). Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology 26 (3): 386–404.
The Bacterial Cytoskeleton. Shih YL, Rothfield L. Microbiol Mol Biol Rev. 2006 Sep;70(3):729-54.
http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&amp;db=pubmed&# 38;amp;dopt=Abstract&amp;list_uids=16959967
chloroplast The organelle of photosynthesis; captures light energy from the sun and uses it with water and carbon dioxide to make food (sugar) for the plant.
cilia (cilium) Made up of extensions of the cell membrane that contain microtubules; involved in movement.
cell wall A rigid layer that is found outside the cell membrane and surrounds the cell; provides structural support and protection.
cytoplasm The gel-like material within the cell that holds the organelles.
cytoskeleton A cellular ”scaffolding” or ”skeleton” that crisscrosses the cytoplasm; helps to maintain cell shape, it holds organelles in place, and for some cells, it enables cell movement.
endoplasmic reticulum (ER) A network of phospholipid membranes that form hollow tubes, flattened sheets, and round sacs; involved in transport of molecules, such as proteins, and the synthesis of proteins and lipids.
flagella (flagellum) Long, thin structures that stick out from the cell membrane; help single-celled organisms move or swim towards food.
Fluid Mosaic Model Model of the structure of cell membranes; proposes that integral membrane proteins are embedded in the phospholipid bilayer; some of these proteins extend all the way through the bilayer, and some only partially across it; also proposes that the membrane behaves like a fluid, rather than a solid.
gene A short segment of DNA that contains information to encode an RNA molecule or a protein strand.
gene expression The process by which the information in a gene is ”decoded” by various cell molecules to produce a functional gene product, such as a protein molecule or an RNA molecule.
Golgi apparatus A large organelle that is usually made up of five to eight cup-shaped, membrane-covered discs called cisternae; modifies, sorts, and packages different sub- stances for secretion out of the cell, or for use within the cell.
www.ck12.org 184
integral membrane proteins Proteins that are permanently embedded within the plasma membrane; involved in channeling or transporting molecules across the membrane or acting as cell receptors.
intermediate filaments Filaments that organize the inside structure of the cell by holding organelles and providing strength.
lipid bilayer A double layer of closely-packed lipid molecules; the cell membrane is a phospholipid bilayer.
lysosome A vesicle that contains powerful digestive enzymes.
membrane protein A protein molecule that is attached to, or associated with the mem- brane of a cell or an organelle.
microfilament Filament made of two thin actin chains that are twisted around one an- other; organizes cell shape; positions organelles in cytoplasm; involved in cell-to-cell and cell-to-matrix junctions.
microtubules Hollow cylinders that make up the thickest of the cytoskeleton structures; made of the protein tubulin, with two subunits, alpha and beta tubulin; involved in organelle and vesicle movement; form mitotic spindles during cell division; involved in cell motility (in cilia and flagella).
mitochondria (mitochondrion) Membrane-enclosed organelles that are found in most eukaryotic cells; called the ”power plants” of the cell because they use energy from organic compounds to make ATP.
multicellular organisms Organisms that are made up of more than one type of cell; have specialized cells that are grouped together to carry out specialized functions.
nucleus The membrane-enclosed organelle found in most eukaryotic cells; contains the genetic material (DNA).
organ A group of tissues that has a specific function or group of functions.
organ system A group of organs that acts together to carry out complex related functions, with each organ focusing on a part of the task.
peripheral membrane proteins Proteins that are only temporarily associated with the membrane; can be easily removed, which allows them to be involved in cell signaling.
peroxisomes Vesicles that use oxygen to break down toxic substances in the cell.
phospholipid A lipid made up of up of a polar, phosphorus-containing head, and two long fatty acid, non-polar ”tails.” The head of the molecule is hydrophilic (water-loving), and the tail is hydrophobic (water-fearing).
plasma membrane Phospholipid bilayer that separates the internal environment of the cell from the outside environment.
ribosomes Organelles made of protein and ribosomal RNA (rRNA); where protein syn- thesis occurs.
selective permeability The ability to allow only certain molecules in or out of the cell; characteristic of the cell membrane; also called the cell membrane.
spontaneous generation The belief that living organisms grow directly from decaying organic substances.
tissue A group of connected cells that has a similar function within an organism.
transport vesicle A vesicle that is able to move molecules between locations inside the cell.
vacuole Membrane-bound organelles that can have secretory, excretory, and storage func- tions; plant cells have a large central vacuole.
vesicle A small, spherical compartment that is separated from the cytosol by at least one lipid bilayer.
How do you think small molecules, or even water, get through the cell membrane?
Is it possible that proteins help in this transport process?
What type of proteins would help with transport? www.ck12.org 186
Identify two ways that molecules and ions cross the plasma membrane.
Distinguish between diffusion and osmosis.
Identify the role of ion channels in facilitated diffusion.
Compare passive and active transport.
Identify the connection between vesicles and active transport.
Compare endocytosis and exocytosis.
Outline the process of cell communication.
Probably the most important feature of a cell’s phospholipid membranes is that they are selectively permeable. A membrane that is selectively permeable has control over what molecules or ions can enter or leave the cell, as shown in Figure 3.27. The permeability of a membrane is dependent on the organization and characteristics of the membrane lipids and proteins. In this way, cell membranes help maintain a state of homeostasis within cells (and tissues, organs, and organ systems) so that an organism can stay alive and healthy.
Figure 3.27: A selectively permeable membrane allows certain molecules through, but not others. (13)
The molecular make-up of the phospholipid bilayer limits the types of molecules that can pass through it. For example, hydrophobic (water-hating) molecules, such as carbon dioxide (CO2) and oxygen (O2), can easily pass through the lipid bilayer, but ions such as calcium (Ca2+) and polar molecules such as water (H2O) cannot. The hydrophobic interior of the phospholipid does not allow ions or polar molecules through because they are hydrophilic, or water loving. In addition, large molecules such as sugars and proteins are too big to pass through the bilayer. Transport proteins within the membrane allow these molecules to cross the membrane into or out of the cell. This way, polar molecules avoid contact with the nonpolar interior of the membrane, and large molecules are moved through large pores.
Every cell is contained within a membrane punctuated with transport proteins that act as channels or pumps to let in or force out certain molecules. The purpose of the transport proteins is to protect the cell’s internal environment and to keep its balance of salts, nutrients, and proteins within a range that keeps the cell and the organism alive.
There are three main ways that molecules can pass through a phospholipid membrane. The first way requires no energy input by the cell and is called passive transport. The second way requires that the cell uses energy to pull in or pump out certain molecules and ions and is called active transport. The third way is through vesicle transport, in which large molecules are moved across the membrane in bubble-like sacks that are made from pieces of the membrane.
Passive transport is a way that small molecules or ions move across the cell membrane without input of energy by the cell. The three main kinds of passive transport are diffusion, osmosis, and facilitated diffusion.
Diffusion is the movement of molecules from an area of high concentration of the molecules to an area with a lower concentration. The difference in the concentrations of the molecules in the two areas is called the concentration gradient. Diffusion will continue until this gradient has been eliminated. Since diffusion moves materials from an area of higher con- centration to the lower, it is described as moving solutes ”down the concentration gradient.” The end result of diffusion is an equal concentration, or equilibrium, of molecules on both sides of the membrane.
If a molecule can pass freely through a cell membrane, it will cross the membrane by diffusion (Figure 3.28).
www.ck12.org 188
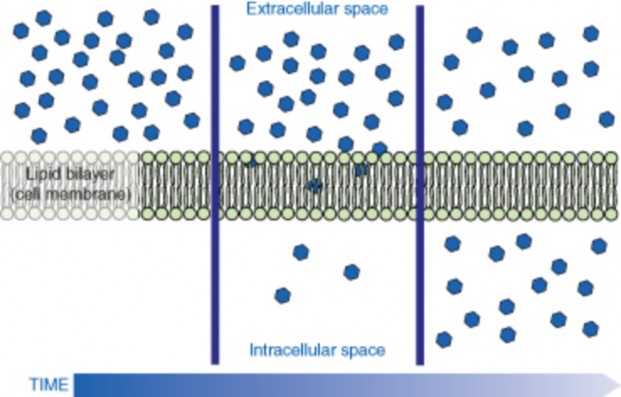
Imagine you have a cup that has 100ml water, and you add 15g of table sugar to the water. The sugar dissolves and the mixture that is now in the cup is made up of a solute (the sugar), that is dissolved in the solvent (the water). The mixture of a solute in a solvent is called a solution.
Imagine now that you have a second cup with 100ml of water, and you add 45 grams of table sugar to the water. Just like the first cup, the sugar is the solute, and the water is the solvent. But now you have two mixtures of different solute concentrations. In comparing two solutions of unequal solute concentration, the solution with the higher solute concentration is hypertonic, and the solution with the lower concentration is hypotonic. Solutions of equal solute concentration are isotonic. The first sugar solution is hypotonic to the second solution. The second sugar solution is hypertonic to the first.
You now add the two solutions to a beaker that has been divided by a selectively permeable membrane. The pores in the membrane are too small for the sugar molecules to pass through, but are big enough for the water molecules to pass through. The hypertonic solution is on one side of the membrane and the hypotonic solution on the other. The hypertonic solution has a lower water concentration than the hypotonic solution, so a concentration gradient of water now exists across the membrane. Water molecules will move from the side of higher water concentration to the side of lower concentration until both solutions are isotonic.
Osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. Water moves into and out of cells by osmosis. If a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol does, and water moves out of the cell until both solutions are isotonic. Cells placed in a hypotonic solution will take in water across their membrane until both the external solution and the cytosol are isotonic.
A cell that does not have a rigid cell wall (such as a red blood cell), will swell and lyse (burst) when placed in a hypotonic solution. Cells with a cell wall will swell when placed in a hypotonic solution, but once the cell is turgid (firm), the tough cell wall prevents any more water from entering the cell. When placed in a hypertonic solution, a cell without a cell wall will lose water to the environment, shrivel, and probably die. In a hypertonic solution, a cell with a cell wall will lose water too. The plasma membrane pulls away from the cell wall as it shrivels. The cell becomes plasmolyzed. Animal cells tend to do best in an isotonic environment, plant cells tend to do best in a hypotonic environment. This is demonstrated in Figure 3.29.
When water moves into a cell by osmosis, osmotic pressure may build up inside the cell. If a cell has a cell wall, the wall helps maintain the cell’s water balance. Osmotic pressure is the main cause of support in many plants. When a plant cell is in a hypotonic environment, the osmotic entry of water raises the turgor pressure exerted against the cell wall until the pressure prevents more water from coming into the cell. At this point the plant cell is turgid.
www.ck12.org 190
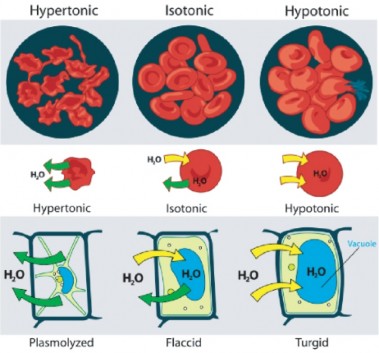
The effects of osmotic pressures on plant cells are shown in Figure 3.30.
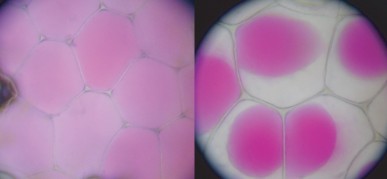
Osmosis can be seen very effectively when potato slices are added to a high concentration of salt solution (hypertonic). The water from inside the potato moves out of the potato cells to the salt solution, which causes the potato cells to lose turgor pressure. The more concentrated the salt solution, the greater the difference in the size and weight of the potato slice after plasmolysis.
The action of osmosis can be very harmful to organisms, especially ones without cell walls. For example, if a saltwater fish (whose cells are isotonic with seawater), is placed in fresh water, its cells will take on excess water, lyse, and the fish will die. Another example of a harmful osmotic effect is the use of table salt to kill slugs and snails.
Organisms that live in a hypotonic environment such as freshwater, need a way to prevent their cells from taking in too much water by osmosis. A contractile vacuole is a type of vacuole that removes excess water from a cell. Freshwater protists, such as the paramecia shown in Figure 3.31, have a contractile vacuole. The vacuole is surrounded by several canals, which absorb water by osmosis from the cytoplasm. After the canals fill with water, the water is pumped into the vacuole. When the vacuole is full, it pushes the water out of the cell through a pore. Other protists, such as members of the genus Amoeba, have contractile vacuoles that move to the surface of the cell when full and release the water into the environment.
www.ck12.org 192
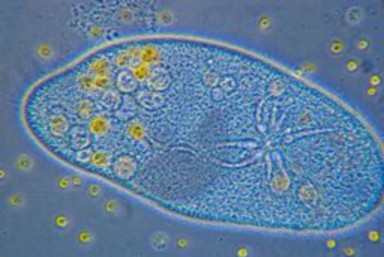
Facilitated diffusion is the diffusion of solutes through transport proteins in the plasma membrane. Facilitated diffusion is a type of passive transport. Even though facilitated diffusion involves transport proteins, it is still passive transport because the solute is moving down the concentration gradient.
As was mentioned earlier, small nonpolar molecules can easily diffuse across the cell mem- brane. However, due to the hydrophobic nature of the lipids that make up cell membranes, polar molecules (such as water) and ions cannot do so. Instead, they diffuse across the mem- brane through transport proteins. A transport protein completely spans the membrane, and allows certain molecules or ions to diffuse across the membrane. Channel proteins, gated channel proteins, and carrier proteins are three types of transport proteins that are involved in facilitated diffusion.
A channel protein, a type of transport protein, acts like a pore in the membrane that lets water molecules or small ions through quickly. Water channel proteins allow water to diffuse across the membrane at a very fast rate. Ion channel proteins allow ions to diffuse across the membrane.
A gated channel protein is a transport protein that opens a ”gate,” allowing a molecule to pass through the membrane. Gated channels have a binding site that is specific for a given molecule or ion. A stimulus causes the ”gate” to open or shut. The stimulus may be chemical or electrical signals, temperature, or mechanical force, depending on the type of gated channel. For example, the sodium gated channels of a nerve cell are stimulated by a chemical signal which causes them to open and allow sodium ions into the cell. Glucose
molecules are too big to diffuse through the plasma membrane easily, so they are moved across the membrane through gated channels. In this way glucose diffuses very quickly across a cell membrane, which is important because many cells depend on glucose for energy.
A carrier protein is a transport protein that is specific for an ion, molecule, or group of substances. Carrier proteins ”carry” the ion or molecule across the membrane by changing shape after the binding of the ion or molecule. Carrier proteins are involved in passive and active transport. A model of a channel protein and carrier proteins is shown in Figure 3.32.
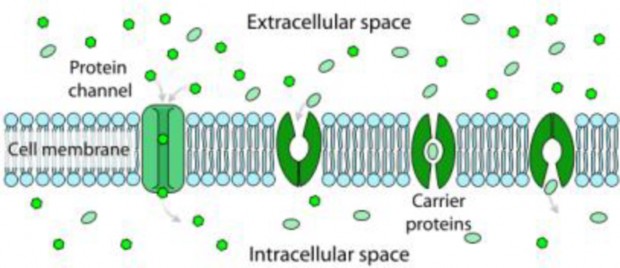
Ions such as sodium (Na+), potassium (K-), calcium (Ca2+), and chloride (Cl-), are im- portant for many cell functions. Because they are polar, these ions do not diffuse through the membrane. Instead they move through ion channel proteins where they are protected from the hydrophobic interior of the membrane. Ion channels allow the formation of a concentration gradient between the extracellular fluid and the cytosol. Ion channels are very specific as they allow only certain ions through the cell membrane. Some ion channels are always open, others are ”gated” and can be opened or closed. Gated ion channels can open or close in response to different types of stimuli such as electrical or chemical signals.
www.ck12.org 194
In contrast to facilitated diffusion which does not require energy and carries molecules or ions down a concentration gradient, active transport pumps molecules and ions against a concentration gradient. Sometimes an organism needs to transport something against a concentration gradient. The only way this can be done is through active transport which uses energy that is produced by respiration (ATP). In active transport, the particles move across a cell membrane from a lower concentration to a higher concentration. Active transport is the energy-requiring process of pumping molecules and ions across membranes ”uphill” against a gradient.
The active transport of small molecules or ions across a cell membrane is generally carried out by transport proteins that are found in the membrane.
Larger molecules such as starch can also be actively transported across the cell mem- brane by processes called endocytosis and exocytosis (discussed later).
Carrier proteins can work with a concentration gradient (passive transport), but some carrier proteins can move solutes against the concentration gradient (from high concentration to low), with energy input from ATP. As in other types of cellular activities, ATP supplies the energy for most active transport. One way ATP powers active transport is by transferring a phosphate group directly to a carrier protein. This may cause the carrier protein to change its shape, which moves the molecule or ion to the other side of the membrane. An example of this type of active transport system, as shown in Figure 3.33, is the sodium-potassium pump, which exchanges sodium ions for potassium ions across the plasma membrane of animal cells.
As is shown in Figure 3.33, three sodium ions bind with the protein pump inside the cell. The carrier protein then gets energy from ATP and changes shape. In doing so, it pumps the three sodium ions out of the cell. At that point, two potassium ions move in from outside the cell and bind to the protein pump. The sodium-potassium pump is found in the plasma membrane of almost every human cell and is common to all cellular life. It helps maintain cell potential and regulates cellular volume. Cystic fibrosis is a genetic disorder that results in a misshapen chloride ion pump. Chloride levels within the cells are not controlled properly, and the cells produce thick mucus. The chloride ion pump is important for creating sweat, digestive juices, and mucus.
The active transport of ions across the membrane causes an electrical gradient to build up across the plasma membrane. The number of positively charged ions outside the cell is
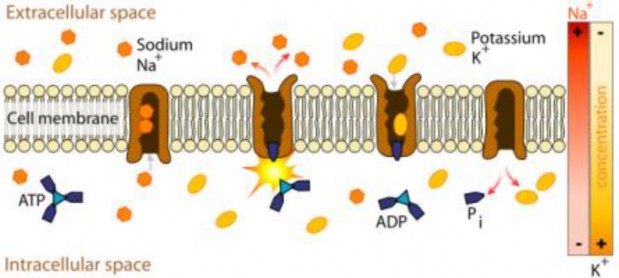
greater than the number of positively charged ions in the cytosol. This results in a relatively negative charge on the inside of the membrane, and a positive charge on the outside. This difference in charges causes a voltage across the membrane. Voltage is electrical potential energy that is caused by a separation of opposite charges, in this case across the membrane. The voltage across a membrane is called membrane potential. Membrane potential is very important for the conduction of electrical impulses along nerve cells.
Because the inside of the cell is negative compared to outside the cell, the membrane potential favors the movement of positively charged ions (cations) into the cell, and the movement of negative ions (anions) out of the cell. So, there are two forces that drive the diffusion of ions across the plasma membrane—a chemical force (the ions’ concentration gradient), and an electrical force (the effect of the membrane potential on the ions’ movement). These two forces working together are called an electrochemical gradient, and will be discussed in detail in the chapter Nervous and Endocrine Systems.
Some molecules or particles are just too large to pass through the plasma membrane or to move through a transport protein. So cells use two other methods to move these macro- molecules (large molecules) into or out of the cell. Vesicles or other bodies in the cytoplasm move macromolecules or large particles across the plasma membrane. There are two types
www.ck12.org 196
of vesicle transport, endocytosis and exocytosis.
Endocytosis is the process of capturing a substance or particle from outside the cell by engulfing it with the cell membrane. The membrane folds over the substance and it be- comes completely enclosed by the membrane. At this point a membrane-bound sac, or vesicle pinches off and moves the substance into the cytosol. There are two main kinds of endocytosis:
Phagocytosis or ”cellular eating,” occurs when the dissolved materials enter the cell. The plasma membrane engulfs the solid material, forming a phagocytic vesicle.
Pinocytosis or ”cellular drinking,” occurs when the plasma membrane folds inward to form a channel allowing dissolved substances to enter the cell, as shown in Figure
3.34. When the channel is closed, the liquid is encircled within a pinocytic vesicle.
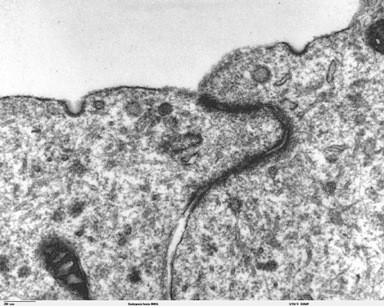
Exocytosis describes the process of vesicles fusing with the plasma membrane and releasing their contents to the outside of the cell, as shown in Figure 3.35. Exocytosis occurs when a cell produces substances for export, such as a protein, or when the cell is getting rid of a waste product or a toxin. Newly made membrane proteins and membrane lipids are moved on top the plasma membrane by exocytosis. For a detailed animation on cellular secretion, see http://vcell.ndsu.edu/animations/constitutivesecretion/first.htm.
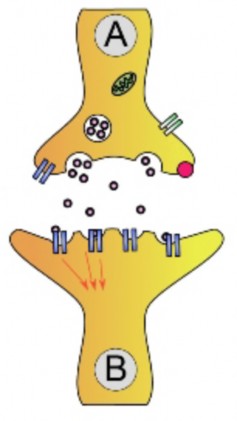
www.ck12.org 198
Homeostasis refers to the balance, or equilibrium within the cell or a body. It is an organ- ism’s ability to keep a constant internal environment. Keeping a stable internal environment requires constant adjustments as conditions change inside and outside the cell. The adjusting of systems within a cell is called homeostatic regulation. Because the internal and external environments of a cell are constantly changing, adjustments must be made continuously to stay at or near the set point (the normal level or range). Homeostasis is a dynamic equi- librium rather than an unchanging state. The cellular processes discussed in this lesson all play an important role in homeostatic regulation. You will learn more about homeostasis in The Human Body chapter.
To survive and grow, cells need to be able to ”talk” with their cell neighbors and be able to detect change in their environment. Talking with neighbors is even more important to a cell if it is part of a multicellular organism. The billions of cells that make up your body need to be able to communicate with each other to allow your body to grow, and to keep you alive and healthy. The same is true for any organism. Cell signaling is a major area of research in biology today. Recently scientists have discovered that many different cell types, from bacteria to plants, use similar types of communication pathways, or cell- signaling mechanisms. This suggests that cell-signaling mechanisms evolved long before the first multicellular organism did.
For cells to be able to signal to each other, a few things are needed:
a signal
a cell receptor, which is usually on the plasma membrane, but can be found inside the cell
a response to the signal
Cells that are communicating may be right next to each other or far apart. The type of chemical signal a cell will send differs depending on the distance the message needs to go. For example, hormones, ions, and neurotransmitters are all types of signals that are sent depending on the distance the message needs to go.
The target cell then needs to be able to recognize the signal. Chemical signals are received by the target cell on receptor proteins. As discussed earlier, most receptor proteins are found in the plasma membrane. Most receptors proteins are found on the plasma membrane, but
some are also found inside the cell. These receptor proteins are very specific for only one particular signal molecule, much like a lock that recognizes only one key. Therefore, a cell has lots of receptor proteins to recognize the large number of cell signal molecules. There are three stages to sending and receiving a cell ”message:” reception, transduction, and response.
Cell-surface receptors are integral proteins—they reach right through the lipid bilayer, span- ning from the outside to the inside of the cell. These receptor proteins are specific for just one kind of signal molecule. The signaling molecule acts as a ligand when it binds to a recep- tor protein. A ligand is a small molecule that binds to a larger molecule. Signal molecule binding causes the receptor protein to change its shape. At this point the receptor protein can interact with another molecule. The ligand (signal molecule) itself does not pass through the plasma membrane.
In eukaryotic cells, most of the intracellular proteins that are activated by a ligand binding to a receptor protein are enzymes. Receptor proteins are named after the type of enzyme that they interact with inside the cell. These enzymes include G proteins and protein kinases, likewise there are G-protein-linked receptors and tyrosine kinase receptors. A kinase is a protein involved in phosphorylation. A G-protein linked receptor is a receptor that works with the help of a protein called a G-protein. A G-protein gets its name from the molecule to which it is attached, guanosine triphosphate (GTP), or guanosine diphosphate (GDP). The GTP molecule is similar to ATP.
Once G proteins or protein kinase enzymes are activated by a receptor protein, they create molecules called second messengers. A second messenger is a small molecule that starts a change inside a cell in response to the binding of a specific signal to a receptor protein. Some second messenger molecules include small molecules called cyclic nucleotides, such as cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). Calcium ions (Ca2+) also act as secondary messengers. Secondary messengers are a part of signal transduction pathways.
A signal-transduction pathway is the signaling mechanism by which a cell changes a signal on it surface into a specific response inside the cell. It most often involves an ordered sequence of chemical reactions inside the cell which is carried out by enzymes and other molecules. In many signal transduction processes, the number of proteins and other molecules participating in these events increases as the process progresses from the binding of the signal. A ”signal cascade” begins. Think of a signal cascade as a chemical domino-effect inside the cell, in which one domino knocks over two dominos, which in turn knock over four dominos, and so on. The advantage of this type of signaling to the cell is that the message from one
www.ck12.org 200
little signal molecule can be greatly amplified and have a dramatic effect.
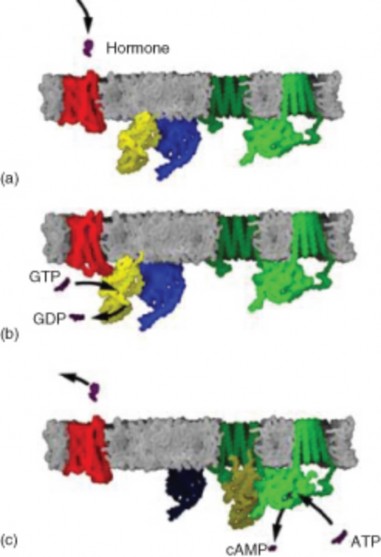
G protein-linked receptors are only found in higher eukaryotes, including yeast, plants, and animals. Your senses of sight and smell are dependent on G-protein linked receptors. The ligands that bind to these receptors include light-sensitive compounds, odors, hormones, and neurotransmitters. The ligands for G-protein linked receptors come in different sizes, from small molecules to large proteins. G protein-coupled receptors are involved in many diseases, but are also the target of around half of all modern medicinal drugs.
The process of how a G-protein linked receptor works is outlined in Figure 3.36.
Table 3.3:
![]()
A ligand such as a hormone (small, pur- ple molecule) binds to the G-linked recep- tor (red molecule). Before ligand binding, the inactive G-protein (yellow molecule) has GDP bound to it.
![]()
The receptor changes shape and activates the G-protein and a molecule of GTP re- places the GDP.
The G-protein moves across the membrane then binds to and activates the enzyme (green molecule). This then triggers the next step in the pathway to the cell’s re- sponse. After activating the enzyme, the G- protein returns to its original position. The second messenger of this signal transduction is cAMP, as shown in C.
![]()
The sensing of the external and internal environments at the cellular level relies on sig- nal transduction. Defects in signal transduction pathways can contribute or lead to many diseases, including cancer and heart disease. This highlights the importance of signal trans- ductions to biology and medicine.
In response to a signal, a cell may change activities in the cytoplasm or in the nucleus that in- clude the switching on or off of genes. Changes in metabolism, continued growth, movement, or death are some of the cellular responses to signals that require signal transduction.
Gene activation leads to other effects, since the protein products of many of the responding genes include enzymes and factors that increase gene expression. Gene expression factors produced as a result of a cascade can turn on even more genes. Therefore one stimulus can trigger the expression of many genes, and this in turn can lead to the activation of many complex events. In a multicellular organism these events include the increased uptake of glucose from the blood stream (stimulated by insulin), and the movement of neutrophils to sites of infection (stimulated by bacterial products). The set of genes and the order in which they are activated in response to stimuli are often called a genetic program.
www.ck12.org 202
Molecules and ions cross the plasma membrane either by passive transport or active the transport.
Passive transport is the movement of molecules across the cell membrane without an input of energy from the cell.
Diffusion is the movement of molecules or ions from an area of high concentration to an area of lower concentration. The molecules keep moving down the concentration gradient until equilibrium is reached.
Osmosis is the diffusion of water molecules across a semipermeable membrane and down a concentration gradient. They can move into or out of a cell, depending on the concentration of the solute.
Active transport moves molecules across a cell membrane from an area of lower con- centration to an area of higher concentration. Active transport requires the use of energy.
The active transport of small molecules or ions across a cell membrane is generally carried out by transport proteins that are found in the membrane.
The sodium-potassium pump is an example of a cell membrane pump. It moves three sodium ions out of the cell and two potassium ions into the cell. The sodium-potassium pump uses ATP.
Endocytosis and exocytosis are active transport mechanisms in which large molecules enter and leave the cell inside vesicles.
In endocytosis, a substance or particle from outside the cell is engulfed by the cell membrane. The membrane folds over the substance and it becomes completely en- closed by the membrane. There are two main kinds of endocytosis: pinocytosis and phagocytosis.
Communication between cells is important for coordinating cell function in an organ- ism. Membrane proteins and vesicles are involved in cellular communication.
Identify the two ways that particles cross the plasma membrane.
How does osmosis differ from diffusion?
Outline how the sodium-potassium pump works.
Are vesicles involved in passive transport? Explain.
What is the difference between endocytosis and exocytosis?
Why is pinocytosis (cellular drinking) a form of endocytosis?
Identify which type of feedback mechanism is most common in homeostasis, and give an example of that type.
Imagine you have discovered a new cell that has not been seen before. How would you go about identifying it based on its structure alone?
Homeostasis can be thought of as a dynamic equilibrium rather than an unchanging
state. Do you agree with this statement? Explain your answer.
This image shows plant cells. The central vacuole of each cell has shrunk and is smaller than normal. What is the likely solute concentration of the cells’ environment which has caused this change?
Biology © 2002 6th Edn. Campbell and Reese. Published by Benjamin Cummings.
active transport The energy-requiring process of pumping molecules and ions across membranes against a concentration gradient.
carrier protein A transport protein that is specific for an ion, molecule, or group of substances; carries the ion or molecule across the membrane by changing shape after the binding of the ion or molecule.
channel protein A transport protein that acts like a pore in the membrane that lets water molecules or small ions through quickly.
contractile vacuole A type of vacuole that removes excess water from a cell. www.ck12.org 204
diffusion The movement of molecules from an area of high concentration of the molecules to an area with a lower concentration.
endocytosis The process of capturing a substance or particle from outside the cell by engulfing it with the cell membrane.
exocytosis The process of vesicles fusing with the plasma membrane and releasing their contents to the outside of the cell.
facilitated diffusion The diffusion of solutes through transport proteins in the plasma membrane.
gated channel protein A transport protein that opens a ”gate,” allowing a molecule to flow through the membrane.
ion channel A protein that transports ions across the membrane by facilitated diffusion.
ligand A small molecule that binds to a larger molecule.
osmosis The diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration.
passive transport A way that small molecules or ions move across the cell membrane without input of energy by the cell.
second messenger A small molecule that starts a change inside a cell in response to the binding of a specific signal to a receptor protein.
selectively permeable The characteristic of the cell membrane that allows certain molecules to pass through the membrane, but not others.
signal-transduction pathway The signaling mechanism by which a cell changes a signal on it surface into a specific response inside the cell; most often involves an ordered sequence of chemical reactions inside the cell which is carried out by enzymes and other molecules.
sodium-potassium pump A carrier protein that moves sodium and potassium ions against large concentration gradients, moves two potassium ions into the cell where potassium levels are high, and pumps three sodium ions out of the cell and into the extracellular fluid.
transport protein A protein that completely spans the membrane, and allows certain molecules or ions to diffuse across the membrane; channel proteins, gated channel proteins, and carrier proteins are three types of transport proteins that are involved in facilitated diffusion.
Next we turn our attention to photosynthesis.
What is photosynthesis?
Where to plants get the ”food” they need?
Where does most of the energy come from?
Wild-type Caenorhabditis elegans. CC-BY.
Robert Hooke, Micrographia,1665. Suber cells and mimosa leaves.. Public Domain.
Raul654,JWSchmidt. http://en.wikipedia.org/wiki/Image:Ostrich_egg.jpg. GNU-FDL,GNU-FDL.
Dr.Ralf Wagner. http://commons.wikimedia.org/wiki/Image:Rotifera.jpg. GFDL.
Mariana Ruiz. http://commons.wikimedia.org/wiki/File: Cell_membrane_detailed_diagram_en.svg. Public Domain.
http://en.wikipedia.org/wiki/Image:Relative_scale.png. Public Domain.
Mariana Ruiz. http://commons.wikimedia.org/wiki/File: Scheme_sodium-potassium_pump-en.svg. Public Domain.
Louisa Howard, Miguel Marin-Padilla. < . Public Domain.
http://en.wikipedia.org/wiki/Image:Antoni_van_Leeuwenhoek.png. Public Domain.
http://en.wikipedia.org/wiki/Image:Animal_cell_structure.svg. Public Domain.
http://commons.wikimedia.org/wiki/File:Phospholipid_structure.png. (a)GNU-FDL and CC-BY-SA 2.5 (b)GNU-FDL.
http://en.wikipedia.org/wiki/Image:Semipermeable_membrane.png. Public Domain.
www.ck12.org 206
http://en.wikipedia.org/wiki/Image:Rhoeo_Discolor_-_Plasmolysis.jpg. GNU-FDL & CC-BY-SA.
http://en.wikipedia.org/wiki/Image:Chloroplast-new.jpg. Public Domain.
Magnus Manske. http://en.wikibooks.org/wiki/Image:Nucleus_ER_golgi.jpg. Public Domain.
http://commons.wikimedia.org/wiki/File:Elektronenmikroskop.jpg. (a)Public Domain (b)GNU-FDL.
CK-12 Foundation. http://commons.wikimedia.org/wiki/File:Diagram_human_cell_nucleus.svg. Public Domain.
http://commons.wikimedia.org/wiki/File: Animal_mitochondrion_diagram_en.svg. (a)Public Domain (b)Public Domain.
http://commons.wikimedia.org/wiki/File:Ribosome_structure.png. GNU-FDL.
CK-12 Foundation. http://schools-wikipedia.org/images/915/91506.png.htm. Public Domain.
http://commons.wikimedia.org/wiki/File: Bronchiolar_area_cilia_cross-sections_2.jpg. Public Domain.
Bensaccount. [Retrieved and modified from
http://en.wikipedia.org/wiki/Image:GPCR_mechanism.png ]. Public Domain.
Mariana Ruiz. http://commons.wikimedia.org/wiki/File: Scheme_facilitated_diffusion_in_cell_membrane-en.svg. Public Domain.
http://en.wikipedia.org/wiki/Image:Tevenphage.png. CC-BY-SA 2.5.
http://commons.wikimedia.org/wiki/Image: Rhoeo_Discolor_-_Plasmolysis.jpg. CC-SA and GFDL.
Dr. Ralf Wagner. Colonial algae of the genus Volvox.. CC-BY.
http://en.wikipedia.org/wiki/Image:Prokaryote_cell_diagram.svg. Public Domain.
MesserWoland and Szczepan1990. http://commons.wikimedia.org/wiki/Image:Biological_cell.svg. GNU-FDL and CC-BY-SA-2.5.
Mariana Ruiz. http://commons.wikimedia.org/wiki/File: Scheme_simple_diffusion_in_cell_membrane-en.svg. Public Domain.
http://commons.wikimedia.org/wiki/File:Chloroplasten.jpg. CC-BY-SA,CC-BY-SA.
Jasper Nance. CC-BY-SA.
Mariana Ruiz. http://commons.wikimedia.org/wiki/File:Plant_cell_structure_svg.svg. Public Domain.
http://commons.wikimedia.org/wiki/Image:Redbloodcells.jpg http://remf.dartmouth.edu/images/bacteriaSEM/source/1.html http://remf.dartmouth.edu/images/algaeSEM/source/1.html http://remf.dartmouth.edu/images/botanicalPollenSEM/source/10.html. CC-BY,Public Domain,Public Domain,Public Domain,Public Domain.
http://en.wikipedia.org/wiki/Image: Turgor_pressure_on_plant_cells_diagram.svg. Public Domain.
Dake. http://en.wikipedia.org/wiki/Image:Synapse_diag1.png. GNU-FDL.
www.ck12.org 208
Identify the kind of energy which powers life.
Contrast the behavior of energy to that of materials in living systems.
Analyze the way in which autotrophs obtain energy and evaluate the importance of autotrophs to energy for all life.
Explain the relationship between autotrophs and heterotrophs.
Discuss the importance of glucose to all life on earth.
Compare the energy-carrying role of ATP to that of glucose.
Explain the roles of chlorophyll and NADPH as sources of energy for life.
Summarize the process of photosynthesis and write out the overall chemical equation for photosynthesis.
Identify reactants, necessary conditions, and products in the chemical equation for photosynthesis.
Describe the roles of chlorophyll and chloroplasts in photosynthesis.
Identify the groups of organisms which are capable of photosynthesis.
Discuss the many reasons photosynthesis is important to humans.
All living things require an ongoing source of energy to do the work of life. You often see energy in action on a large scale: a whale breaches, apple blossoms swell and burst, a firefly glows, or an inky cap mushrooms overnight. However, energy works constantly to maintain
life on a very small scale as well. Inside each cell of every organism, energy assembles chains of information and constructs cellular architecture. It moves tiny charged particles and giant protein molecules. Moreover, it builds and powers cell systems for awareness, response, and reproduction. All life’s work requires energy.
Physics tells us that organized systems, such as living organisms, tend to disorder without a constant input of energy. You have direct, everyday experience with this law of nature: after a week of living in your room, you must spend energy in order to return it to its previous, ordered state. Tides and rain erode your sandcastles, so you must work to rebuild them. And your body, after a long hike or big game, must have more fuel to keep going. Living things show amazing complexity and intricate beauty, but if their source of energy fails, they suffer injury, illness, and eventually death.
Physics also tells us that, although energy can be captured or transformed, it inevitably degrades, becoming heat, a less useful form of energy. This is why organisms require a constant input of energy; the work they must do uses up the energy they take in. Energy, unlike materials, cannot be recycled. The story of life is a story of energy flow – its capture, transformation, use for work, and loss as heat.
Energy, the ability to do work, can take many forms: heat, nuclear, electrical, magnetic, light, and chemical energy. Life runs on chemical energy - the energy stored in covalent bonds between atoms in a molecule. Where do organisms get their chemical energy? That depends…
Living organisms obtain chemical energy in one of two ways.
Autotrophs, shown in Figure 4.1, store chemical energy in carbohydrate food molecules they build themselves. Food is chemical energy stored in organic molecules. Food provides both the energy to do work and the carbon to build bodies. Because most autotrophs transform sunlight to make food, we call the process they use photosynthesis. Only three groups of organisms - plants, algae, and some bacteria - are capable of this life-giving energy transformation. Autotrophs make food for their own use, but they make enough to support other life as well. Almost all other organisms depend absolutely on these three groups for the food they produce. The producers, as autotrophs are also known, begin food chains which feed all life. Food chains will be discussed in the Principles of Ecology chapter.
Heterotrophs cannot make their own food, so they must eat or absorb it. For this reason, heterotrophs are also known as consumers. Consumers include all animals and fungi and many protists and bacteria. They may consume autotrophs, or other heterotrophs or organic molecules from other organisms. Heterotrophs show great diversity and may appear far more fascinating than producers. But heterotrophs are limited by our utter dependence on
www.ck12.org 210
those autotrophs which originally made our food. If plants, algae, and autotrophic bacteria vanished from earth, animals, fungi, and other heterotrophs would soon disappear as well. All life requires a constant input of energy. Only autotrophs can transform that ultimate, solar source into the chemical energy in food which powers life, as shown in Figure 4.2.

Figure 4.1: Photosynthetic autotrophs, which make food for more than 99% of the organisms on earth, include only three groups of organisms: plants such as the redwood tree (a), algae such as kelp (b), and certain bacteria like this Anabaena (c). (14)
Photosynthesis provides over 99 percent of the energy supply for life on earth. A much smaller group of autotrophs - mostly bacteria in dark or low-oxygen environments - produce food using the chemical energy stored in inorganic molecules such as hydrogen sulfide, ammonia, or methane. While photosynthesis transforms light energy to chemical energy, this alternate method of making food transfers chemical energy from inorganic to organic molecules. It is therefore called chemosynthesis, and is characteristic of the tubeworms shown in Figure 4.3. Some of the most recently discovered chemosynthetic bacteria inhabit deep ocean hot water vents or “black smokers.” There, they use the energy in gases from the Earth’s interior to produce food for a variety of unique heterotrophs: giant tube worms, blind shrimp, giant white crabs, and armored snails. Some scientists think that chemosynthesis may support life below the surface of Mars, Jupiter’s moon, Europa, and other planets as well. Ecosystems based on chemosynthesis may seem rare and exotic, but they too illustrate the absolute dependence of heterotrophs on autotrophs for food.
You know that the fish you had for lunch contained protein molecules. But do you know that the atoms in that protein could easily have formed the color in a dragonfly’s eye, the heart of a water flea, and the whiplike tail of a Euglena before they hit your plate as sleek fish muscle? As you learned above, food consists of organic (carbon-containing) molecules which store energy in the chemical bonds between their atoms. Organisms use the atoms of food molecules to build larger organic molecules including proteins, DNA, and fats and
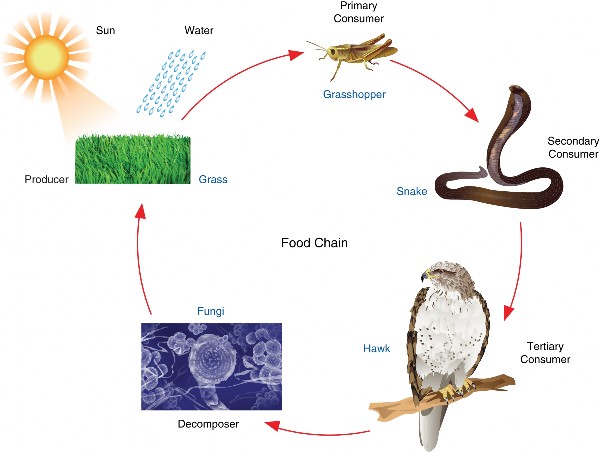
www.ck12.org 212

use the energy in food to power life processes. By breaking the bonds in food molecules, cells release energy to build new compounds. Although some energy dissipates as heat at each energy transfer, much of it is stored in the newly made molecules. Chemical bonds in organic molecules are a reservoir of the energy used to make them. Fueled by the energy from food molecules, cells can combine and recombine the elements of life to form thousands of different molecules. Both the energy (despite some loss) and the materials (despite being reorganized) pass from producer to consumer – perhaps from algal tails, to water flea hearts, to dragonfly eye colors, to fish muscle, to you!
The process of photosynthesis, which usually begins the flow of energy through life, uses many different kinds of energy-carrying molecules to transform sunlight energy into chemical energy and build food.
Some carrier molecules hold energy briefly, quickly shifting it like a hot potato to other molecules. This strategy allows energy to be released in small, controlled amounts. An example is chlorophyll, the green pigment present in most plants which helps convert solar energy to chemical energy. When a chlorophyll molecule absorbs light energy, electrons are excited and “jump” to a higher energy level. The excited electrons then bounce to a series of carrier molecules, losing a little energy at each step. Most of the “lost” energy powers some small cellular task, such as moving ions across a membrane or building up another molecule. Another short-term energy carrier important to photosynthesis, NADPH, holds chemical energy a bit longer but soon “spends” it to help to build sugar.
Two of the most important energy-carrying molecules are glucose and ATP, adenosine triphosphate. These are nearly universal fuels throughout the living world and both are also key players in photosynthesis, as shown below.
A molecule of glucose, which has the chemical formula C6H12O6, carries a packet of chemical energy just the right size for transport and uptake by cells. In your body, glucose is the “deliverable” form of energy, carried in your blood through capillaries to each of your 100 trillion cells. Glucose is also the carbohydrate produced by photosynthesis, and as such is the near-universal food for life.
ATP molecules store smaller quantities of energy, but each releases just the right amount to actually do work within a cell. Muscle cell proteins, for example, pull each other with the energy released when bonds in ATP break open (discussed below). The process of photosynthesis also makes and uses ATP - for energy to build glucose! ATP, then, is the useable form of energy for your cells.
Glucose is the energy-rich product of photosynthesis, a universal food for life. It is also the primary form in which your bloodstream delivers energy to every cell in your body. The six carbons are numbered.
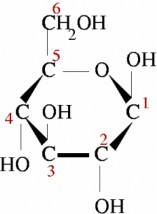
Why do we need both glucose and ATP? Why don’t plants just make ATP and be done with it? If energy were money, ATP would be a quarter. Enough money to operate a parking meter or washing machine. Glucose would be a dollar bill (or $10) – much easier to carry around in your wallet, but too large to do the actual work of paying for parking or washing. Just as we find several denominations of money useful, organisms need several “denominations” of energy – a smaller quantity for work within cells, and a larger quantity for stable storage, transport, and delivery to cells.
Let’s take a closer look at a molecule of ATP. Although it carries less energy than glucose, its structure is more complex. “A” in ATP refers to the majority of the molecule – adenosine
a combination of a nitrogenous base and a five-carbon sugar. “T” and “P” indicate the three phosphates, linked by bonds which hold the energy actually used by cells. Usually, only the outermost bond breaks to release or spend energy for cellular work.
An ATP molecule, shown below, is like a rechargeable battery: its energy can be used by the cell when it breaks apart into ADP (adenosine diphosphate) and phosphate, and then the “worn-out battery” ADP can be recharged using new energy to attach a new phosphate
www.ck12.org 214
and rebuild ATP. The materials are recyclable, but recall that energy is not!
How much energy does it cost to do your body’s work? A single cell uses about 10 million ATP molecules per second, and recycles all of its ATP molecules about every 20-30 seconds.
A red arrow shows the bond between two phosphate groups in an ATP molecule. When this bond breaks, its chemical energy can do cellular work. The resulting ADP molecule is recycled when new energy attaches another phosphate, rebuilding ATP.
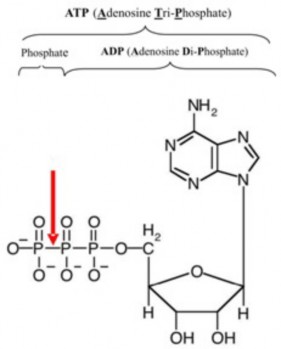
Keep these energy-carrying molecules in mind as we look more carefully at the process which originally captures the energy to build them: photosynthesis. Recall that it provides nearly all of the food (energy and materials) for life. Actually, as you will see, we are indebted to photosynthesis for even more than just the energy and building blocks for life.
What do pizza, campfires, dolphins, automobiles, and glaciers have in common? In the following section, you’ll learn that all five rely on photosynthesis, some in more ways than one. Photosynthesis is often considered the most important chemical reaction for life on earth. Let’s delve into how this process works and why we are so indebted to it.
Photosynthesis involves a complex series of chemical reactions, each of which convert one substance to another. These reactions taken as a whole can be summarized in a single
symbolic representation – as shown in the chemical equation below.
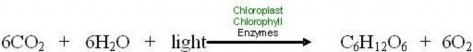
We can substitute words for the chemical symbols. Then the equation appears as below.
![]()
Like all chemical equations, this equation for photosynthesis shows reactants connected by plus signs on the left and products, also connected by plus signs, on the right. An arrow indicating the process or chemical change leads from the reactants to the products, and conditions necessary for the chemical reaction are written above the arrow. Note that the same kinds of atoms, and number of atoms, are found on both sides of the equation, but the kinds of compounds they form change.
You use chemical reactions every time you cook or bake. You add together ingredients (the reactants), place them in specific conditions (often heat), and enjoy the results (the products). A recipe for chocolate chip cookies written in chemical equation form is shown below.
Compare this familiar recipe to photosynthesis below.
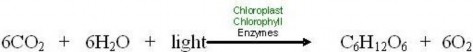
The equation shows that the “ingredients” for photosynthesis are carbon dioxide, water, and light energy. Plants, algae, and photosynthetic bacteria take in light from the sun, molecules of carbon dioxide from the air, and water molecules from their environment and combine these reactants to produce food (glucose).
Of course, light, carbon dioxide, and water mix in the air even without plants. But they do not chemically change to make food without very specific necessary conditions which are found only in the cells of photosynthetic organisms. Necessary conditions include:
enzymes - proteins which speed up chemical reactions without the heat required for www.ck12.org 216
cooking
chlorophyll - a pigment which absorbs light
chloroplasts - organelles whose membranes embed chlorophyll, accessory pigments, and enzymes in patterns which maximize photosynthesis
Within plant cells or algal cells, chloroplasts organize the enzymes, chlorophyll, and accessory pigment molecules necessary for photosynthesis.
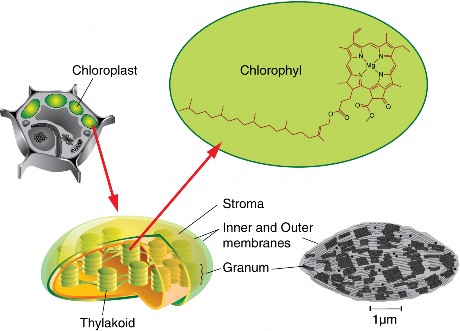
When the reactants meet inside chloroplasts, or the very similar cells of blue-green bacteria, chemical reactions combine them to form two products: energy-rich glucose molecules and molecules of oxygen gas. Photosynthetic organisms store the glucose (usually as starch) and release the oxygen gas into the atmosphere as waste.
Let’s review the chemical equation for photosynthesis once more, this time at the level of atoms as in the equation below.
![]()
Look closely at its primary purpose: storing energy in the chemical bonds of food molecules. The source of energy for food is sunlight energy. The source of carbon atoms for the food molecules is carbon dioxide from the air, and the source of hydrogen atoms is water. Inside the cells of plants, algae, and photosynthetic bacteria, chlorophyll, and enzymes use the light energy to rearrange the atoms of the reactants to form the products, molecules of glucose and oxygen gas. Light energy is thus transformed into chemical energy, stored in the bonds
which bind six atoms each of carbon and oxygen to twelve atoms of hydrogen – forming a molecule of glucose. This energy rich carbohydrate molecule becomes food for the plants, algae, and bacteria themselves as well as for the heterotrophs which feed on them.
One last detail: why do “6”s precede the CO2, H2O, and O2? Look carefully, and you will see that this “balances” the equation: the numbers of each kind of atom on each side of the arrow are equal. Six molecules each of CO2 and H2O make 1 molecule of glucose and 6 molecules of oxygen gas.
All organisms require a constant input of energy to do the work of life.
Energy cannot be recycled, so the story of life is a story of energy flow – its capture, transformation, use for work, and loss as heat.
Life runs on chemical energy.
Food is chemical energy stored in organic molecules.
Food provides both the energy to do life’s work and the carbon to build life’s bodies.
The carbon cycles between organisms and the environment, but the energy is “spent” and must be replaced.
Organisms obtain chemical energy in one of two ways.
Autotrophs make their own carbohydrate foods, transforming sunlight in photosyn- thesis or transferring chemical energy from inorganic molecules in chemosynthesis.
Heterotrophs consume organic molecules originally made by autotrophs.
All life depends absolutely upon autotrophs to make food molecules.
The process of photosynthesis produces more than 99% of all food for life, forming the foundation of most food chains.
Only three groups of organisms – plants, algae, and some bacteria – carry out the process of photosynthesis.
All organisms use similar energy-carrying molecules for food and to carry out life processes.
Glucose (C6H12O6,) is a nearly universal fuel delivered to cells, and the primary product of photosynthesis.
www.ck12.org 218
ATP molecules store smaller amounts of energy and are used within cells to do work.
Chlorophyll and NADPH molecules hold energy temporarily during the process of photosynthesis.
The chemical equation below summarizes the many chemical reactions of photosynthesis.
![]()
The equation states that the reactants (carbon dioxide, water and light), in the presence of chloroplasts, chlorophyll and enzymes, yield two products, glucose and oxygen gas.
Chlorophyll is a pigment that absorbs sunlight energy.
Chloroplasts are the organelles within plant and algal cells that organize enzymes and pigments so that the chemical reactions proceed efficiently.
In the process of photosynthesis, plants, algae, and blue green bacteria absorb sunlight energy and use it to change carbon dioxide and water into glucose and oxygen gas.
Glucose contains stored chemical energy and provides food for the organisms that produce it and for many heterotrophs.
Photosynthesized carbohydrates (represented here by glucose) make up the wood we burn and (over hundreds of millions of years) the coal, oil, and gas we now use as fossil fuels.
Most of the oxygen gas is waste for the organisms which produce it.
Both CO2 consumed and O2 produced affect the composition of earth’s atmosphere; before photosynthesis evolved, oxygen was not part of the atmosphere.
Compare the behavior of energy to the behavior of matter in living systems.
Water and carbon dioxide molecules are reactants in the process of photosynthesis. Does this mean they are “food” for plants, algae, and blue-green bacteria? Use the definition of “food” to answer this question.
Compare autotrophs to heterotrophs, and describe the relationship between these two groups of organisms.
Name and describe the two types of food making found among autotrophs, and give an example of each. Which is quantitatively more important to life on earth?
Trace the flow of energy through a typical food chain (describing ”what eats what”), including the original source of that energy and its ultimate form after use. Under- line each form of energy or energy-storing molecule, and boldface each process which transfers or transforms energy.
Trace the pathway that carbon atoms take through a typical food chain, beginning with their inorganic source.
The fact that all organisms use similar energy-carrying molecules shows one aspect of the grand ”Unity of Life.” Name two universal energy-carrying molecules, and explain why most organisms need both carriers rather than just one.
A single cell uses about 10 million ATP molecules per second. Explain how cells use the energy and recycle the materials in ATP.
Discuss the importance of photosynthesis to humans in terms of food, fuel, and atmo- sphere. In what ways could you affect the process of photosynthesis to conserve these benefits?
Using symbols, write the overall chemical equation for photosynthesis, labeling the reactants, necessary conditions, and products. Then write two complete sentences which trace the flow of (1) energy and (2) atoms from reactants to products.
Graham Kent, “Light Reactions in Photosynthesis” Animation. Bio 231 Cell Biology Lab, October 2004. Available on the Web at:
http://www.science.smith.edu/departments/Biology/Bio231/ltrxn.html.
Illustrator: Thomas Porostocky; Writer: Lee Billings; Map data adapted from MODIS observations by NASA’s Terra and Aqua satellites; Graph data and reference: Biology, 4th ed., Neil A. Campbell, Benjamin/Cummings Publishing Company, 1996. “Crib Sheet #10, Photosynthesis.” Seed Magazine, August 2007. Available on the Web at:
John Mynett, “Photosynthesis Animations.” Biology4All, 01 January 2002. Available on the Web at:
http://www.biology4all.com/resources_library/details.asp?ResourceID=43
Kenneth R. Spring, Thomas J. Fellers, and Michael W. Davidson, “Introduction to Light and Energy.” Molecular Expressions Optical Microscopy Primer. The Physics of Light and Energy, Last modified Aug 23, 2005. Available on the Web at
http://micro.magnet.fsu.edu/primer/lightandcolor/lightandenergyintro.html.
“Photosynthesis,” “Electron Transport Chain” and “ATP Synthase” Animations. Vir- tual Cell Animation Collection, Molecular and Cellular Biology Learning Center, no date given. Available on the Web at:
http://vcell.ndsu.nodak.edu/animations/photosynthesis/index.htm.
ATP Adenosine triphosphate, the energy-carrying molecule used by cells to do work.
autotroph An organism capable of transforming one form of energy – usually light – into the food, or stored chemical energy, they need to do work.
www.ck12.org 220
chemosynthesis Process by which a type of autotroph makes food using chemical energy in inorganic molecules.
chlorophyll The primary pigment of photosynthesis.
chloroplast The organelle in plant and algal cells where photosynthesis takes place.
consumers Heterotrophs, which must eat or absorb organic food molecules because they are incapable of producing them.
energy The ability to do work.
food Organic (carbon-containing) molecules which store energy in the chemical bonds be- tween their atoms.
food chain A pathway which traces energy flow from producers through consumers. glucose The carbohydrate product of photosynthesis; serves as the universal fuel for life. heat Thermal energy, the energy of vibrations in molecules – the “lowest” form of energy,
which cannot easily be used for useful work.
heterotrophs Organisms which must consume organic molecules because they are inca- pable of synthesizing the food, or stored chemical energy, they need to work.
inorganic molecules Molecules which do not contain carbon (with a few exceptions such as carbon dioxide) and are not necessarily made by living organisms.
NADPH An energy carrier molecule produced in the light reactions of photosynthesis and used to build sugar in the Calvin cycle.
organic molecule A molecule which contains carbon, made by living organisms; examples include carbohydrates, lipids, and proteins.
photosynthesis The process by which plants, algae, and some bacteria transform sunlight into chemical energy and use it to produce carbohydrate food and oxygen for almost all life.
producer An autotroph, capable of synthesizing food molecules; forms basis of food chains.
Why do some people describe photosynthesis by plants as “making food from thin air”?
Before we conclude this analysis of “the most important chemical reaction for life on Earth,” solidify your understanding of its importance by returning to the pizza, camp- fires, dolphins, automobiles, and glaciers. Can you connect them all to the chemical equation for photosynthesis (Figure 4.4)?
You’ll be able to make more connections after studying the next chapter on cellular respiration. Can you already connect carbon dioxide and oxygen to automobiles?
www.ck12.org 222
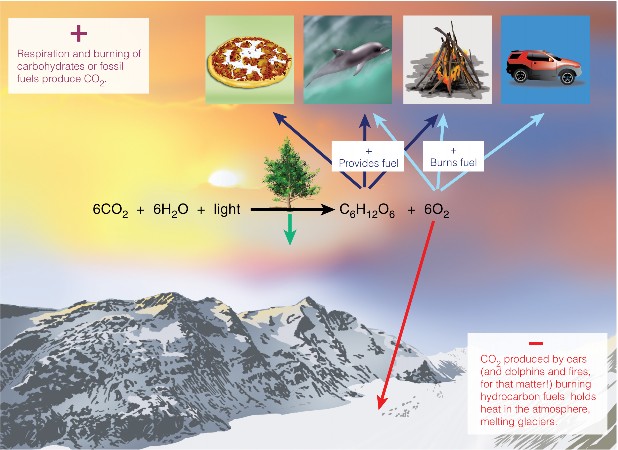
Understand that hundreds of years of scientific exploration have contributed to our understanding of photosynthesis.
Explain the contributions of Van Helmont, Priestley, and Melvin Calvin to our under- standing of photosynthesis.
Describe the structure and function of chloroplasts, thylakoids, and pigments.
Explain how electron carrier molecules form electron transport chains.
Trace the flow of energy and materials through the Light Reactions, including chemios- mosis.
Trace the flow of energy and materials through The Calvin Cycle.
Compare and contrast C-3, C-4, and CAM pathways for carbon fixation.
Life requires photosynthesis for fuel and for the oxygen to burn that fuel. Since the Industrial Revolution (late 18th and early 19th centuries), we humans have relied on products of ancient photosynthesis for enormous quantities of fossil fuel energy. And, knowingly or not, we have also benefited from photosynthesis to remove the carbon dioxide produced when we burn those fuels. So it may not surprise you that biologists have studied this critical process in great detail. The goals of this lesson are:
to discuss how scientists have explored this most important chemical reaction for life on earth
to encourage you to appreciate just a little of its intricate beauty, and
to understand how your own decisions and actions can influence the process of photo- synthesis.
You’ve learned that a single chemical reaction represents the overall process of photosynthesis as demonstrated in the equation below.
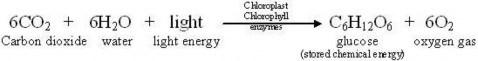
Although photosynthesis may seem straightforward in this form, such simplicity is deceiving for two reasons. First, the equation above summarizes dozens of individual chemical reactions
www.ck12.org 224
involving many intermediate compounds. And second, just discovering major players like CO2 and O2 was challenging, because our ordinary senses cannot detect these molecules in “thin air!”
How do we know that the chemical reaction in photosynthesis really happens? Two famous historical experiments help us begin to understand this process.

Figure 4.5: In the 17th century, Jan Van Helmont, a Flemish chemist, physiologist, and physician, weighed and potted a willow tree, showing that plants do not get food from the soil. (15)
In the 17th century, people who thought about it at all assumed that plants get their food from the soil. Many people, encouraged by sellers of “plant food,” still do. In 1638, Jan Baptist Van Helmont planted a 5 pound willow tree, like the one shown in Figure 4.5, in a 200 pound tub of soil. After 5 years of watering the plant, he weighed both again. The willow had gained over 160 pounds, but the soil had lost only 2 ounces. Van Helmont concluded that plants do not get their materials from soil, and inferred that they grow using materials from water (which he did not measure). As you know now, he was half right. Although soil provides important nutrients to plants, it supplies neither the energy nor the vast majority of the materials to build the plant. We must excuse him, because no one in the 17th century knew that carbon atoms form the basis of life, or that they float around in air in the form of carbon dioxide.
In the late 1770s, minister and natural philosopher Joseph Priestley burned a candle in a jar of air and observed that the candle burned out long before it ran out of wax. A similar
experiment with a mouse resulted in the mouse’s death. Priestley suggested that animals, like candles, “injure” the air. Adding a mint plant, as shown in Figure 4.6, however, “restored” the air which had been “injured” by the mouse or the candle. Only later, after many chemistry experiments, did Priestley publish his discovery of “dephlogisticated air.” But in his studies of mice, plants, and candles, he had shown that plants produce, and animals consume, oxygen gas.
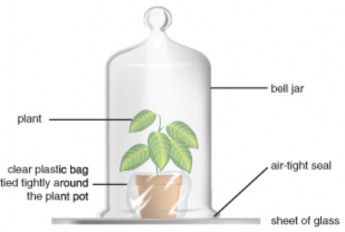
Figure 4.6: Joseph Priestly’s bell jar experiment. (8)
During the 20th century, we learned that photosynthesis involves much more than just the three reactants, the three necessary conditions, and the two products shown in the equation. Using powerful microscopes, we’ve narrowed the process to one type of organelle within the plant – the chloroplast. In the next section, you will learn in more detail just how plants, algae, and photosynthetic bacteria make food for us all “from thin air.” First, let’s look at the organelle in which the drama of photosynthesis takes place and meet some of the key actors.
For a detailed animation of the complete photosynthesis process, see http://vcell.ndsu. edu/animations/photosynthesis/first.htm.
If you examine a single leaf of the aquatic plant Elodea, shown in Figure 4.7, under a micro- scope, you will see within each cell dozens of small green ovals. These are chloroplasts, the organelles which conduct photosynthesis in plants and algae. Chloroplasts closely resemble some types of bacteria and even contain their own circular DNA and ribosomes. In fact, the endosymbiotic theory holds that chloroplasts were once independently living bacteria (prokaryotes). So when we say that photosynthesis occurs within chloroplasts, we speak not
www.ck12.org 226
only of the organelles within plants and algae, but also of some bacteria – in other words, virtually all photosynthetic autotrophs.

Figure 4.7: Elodea (above), like all plants and algae, consists of cells which contain organelles called chloroplasts (green ovals in the microphotograph below). If you look carefully at living cells through a microscope, you may see the chloroplasts moving slowly around the cell edges. The plant itself may not move, but this cyclosis hints at all the action within plant cells. (7)
Both chloroplasts and photosynthetic bacteria contain neat stacks (grana) of flattened sac- shaped membrane compartments (thylakoids), made in turn of elaborate and highly or- ganized patterns of molecules which conduct photosynthesis, as shown in Figure 4.8. In addition to enzymes, two basic types of molecules - pigments and electron carriers – are key players.
Pigment molecules, often arranged together with proteins in large, complex photosystems, absorb specific wavelengths of light energy and reflect others; therefore, they appear colored. The most common photosynthetic pigment is chlorophyll, which absorbs blue-violet and red wavelengths of light, and reflects green (Figure 4.9 and Figure 4.10). Accessory pigments absorb other colors of light and then transfer the energy to chlorophyll. These include xanthophylls (yellow) and carotenoids (orange).
Electron carrier molecules are usually arranged in electron transport chains (ETCs). These accept and pass along energy-carrying electrons in small steps (Figure 4.11). In this way, they produce ATP and NADPH, which temporarily store chemical energy. Electrons
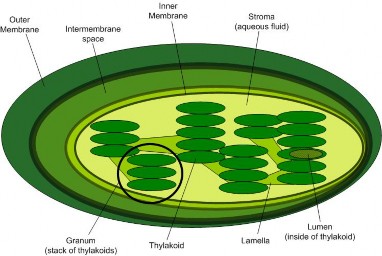
in transport chains behave much like a ball bouncing down a set of stairs – a little energy is lost with each bounce. However, the energy “lost” at each step in an electron transport chain accomplishes a little bit of work, which eventually results in the synthesis of ATP.
Now that you’ve met some of the key players and explored the theater, let’s put them together to see how the process unfolds. We will divide the process into two basic sets of reactions, known as the light reactions and the Calvin cycle, which uses carbon dioxide. As you study the details, refer frequently to the chemical equation of photosynthesis. In the first stage, you’ll discover how chloroplasts transform light energy, and why we owe our ability to breathe to plants!
Every second, the sun fuses over 600 million tons of hydrogen into 596 tons of helium, converting over 4 tons of helium (4.3 billion kg) into light and heat energy. Countless tiny packets of that light energy travel 93 million miles (150 million km) through space, and about 1% of the light which reaches the Earth’s surface participates in photosynthesis. Light is the source of energy for photosynthesis, and the first set of reactions which begin the process requires light – thus the name, Light Reactions, or Light-dependent Reactions.
www.ck12.org 228
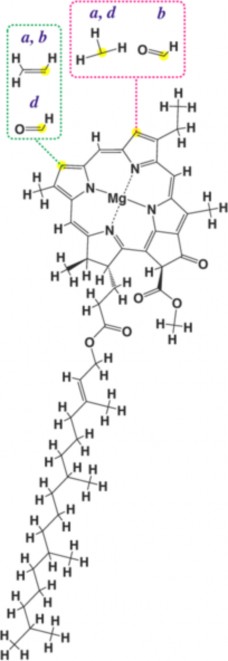
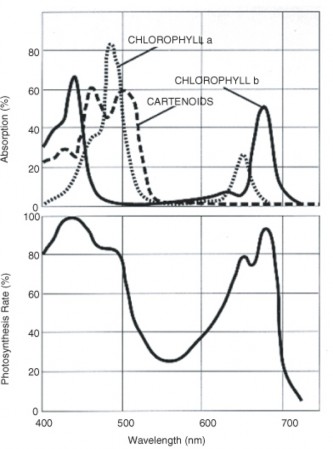
www.ck12.org 230
When light strikes chlorophyll (or an accessory pigment) within the chloroplast, it energizes electrons within that molecule. These electrons jump up to higher energy levels; they have absorbed or captured, and now carry, that energy. High-energy electrons are “excited.” Who wouldn’t be excited to hold the energy for life?
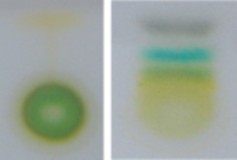
The excited electrons leave chlorophyll to participate in further reactions, leaving the chloro- phyll “at a loss”; eventually they must be replaced. That replacement process also requires light, working with an enzyme complex to split water molecules. In this process of photoly- sis (“splitting by light”), H2O molecules are broken into hydrogen ions, electrons, and oxygen atoms. The electrons replace those originally lost from chlorophyll. Hydrogen ions and the high-energy electrons from chlorophyll will carry on the energy transformation drama after the Light Reactions are over.
The oxygen atoms, however, form oxygen gas, which is a waste product of photosynthesis (Figure 4.12). The oxygen given off supplies most of the oxygen in our atmosphere. Be- fore photosynthesis evolved, Earth’s atmosphere lacked oxygen altogether, and this highly reactive gas was toxic to the many organisms living at the time. Something had to change! Most contemporary organisms rely on oxygen for efficient respiration. So plants don’t just “restore” the air, as Priestley suggested. They also had a major role in creating it!
To summarize, chloroplasts “capture” sunlight energy in two ways. Light “excites” electrons in pigment molecules, and light provides the energy to split water molecules, providing more electrons as well as hydrogen ions.
Now let’s follow those excited electrons…

Excited electrons which have absorbed light energy are unstable. However, the highly or- ganized electron carrier molecules embedded in chloroplast membranes order the flow of these electrons, directing them through electron transport chains (ETCs). At each transfer, small amounts of energy released by the electrons are captured and put to work or stored. Some is also lost as heat with each transfer, but overall the light reactions are extremely efficient at capturing light energy and transforming it to chemical energy.
Two sequential transport chains harvest the energy of excited electrons, as shown in Figure
4.13.
First, they pass down an ETC which captures their energy and uses it to pump hydrogen ions by active transport into the thylakoids. These concentrated ions store potential energy by forming a chemiosmotic or electrochemical gradient – a higher concen- tration of both positive charge and hydrogen inside the thylakoid than outside. (The gradient formed by the H+ ions is known as a chemiosmotic gradient.) Picture this energy buildup of H+ as a dam holding back a waterfall. Like water flowing through a hole in the dam, hydrogen ions “slide down” their concentration gradient through a membrane protein which acts as both ion channel and enzyme. As they flow, the ion channel/enzyme ATP synthase uses their energy to chemically bond a phosphate group to ADP, making ATP.
Light re-energizes the electrons, and they travel down a second electron transport chain (ETC), eventually bonding hydrogen ions to NADP+ to form a more stable energy storage molecule, NADPH. NADPH is sometimes called “hot hydrogen,” and its en- ergy and hydrogen atoms will be used to help build sugar in the second stage of
www.ck12.org 232
photosynthesis.
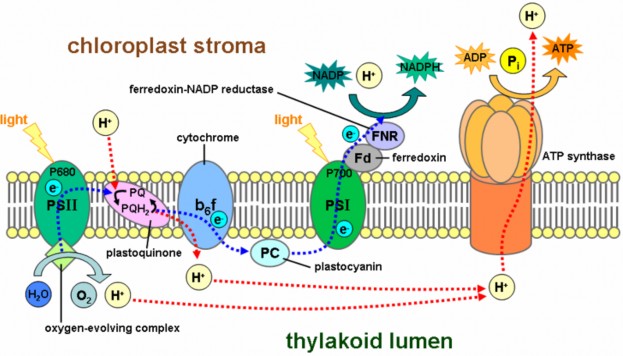
−→ −→ −→
−→ −→
Figure 4.13: Membrane architecture: The large colored carrier molecules form electron trans- port chains which capture small amounts of energy from excited electrons in order to store it in ATP and NADPH. Follow the energy pathways: light electrons NADPH (blue line) and light electrons concentrated H+ ATP (red line). Note the intricate organization of the chloroplast. (10)
NADPH and ATP molecules now store the energy from excited electrons – energy which was originally sunlight – in chemical bonds. Thus chloroplasts, with their orderly arrangement of pigments, enzymes, and electron transport chains, transform light energy into chemical energy. The first stage of photosynthesis – light-dependent reactions or simply “light reactions” – is complete.
You’ve learned that the first, light-dependent stage of photosynthesis uses two of the three reactants - water and light - and produces one of the products - oxygen gas (a waste product of this process). All three necessary conditions are required – chlorophyll pigments, the chloroplast “theater,” and enzyme catalysts. The first stage transforms light energy into
chemical energy, stored to this point in molecules of ATP and NADPH. Look again at the overall equation below. What is left?

Waiting in the wings is one more reactant – carbon dioxide, and yet to come is the star product which is food for all life – glucose. These key players perform in the second act of the photosynthesis drama, in which food is “made from thin air!”
The second stage of photosynthesis can proceed without light, so its steps are sometimes called “light-independent” or “dark” reactions. Many biologists honor the scientist, Melvin Calvin, who won a 1961 Nobel Prize for working out this complex set of chemical reactions, naming it the Calvin Cycle.
The Calvin Cycle has two parts. First carbon dioxide is ”fixed.” Then ATP and NADPH from the Light Reactions provide energy to combine the fixed carbons to make sugar.
Why does carbon dioxide need to be fixed? Was it ever broken?
Life on Earth is carbon-based. Organisms need not only energy but also carbon atoms for building bodies. For nearly all life, the ultimate source of carbon is carbon dioxide (CO2), an inorganic molecule. CO2, as you saw in Figure 4.14, makes up .038% of the Earth’s atmosphere.
Animals and most other heterotrophs cannot take in CO2 directly. They must eat other organisms or absorb organic molecules to get carbon. Only autotrophs can build low- energy inorganic CO2 into high-energy organic molecules like glucose. This process is carbon fixation.
Plants have evolved three pathways for carbon fixation.
The most common pathway combines one molecule of CO2 with a 5-carbon sugar called ribu- lose biphosphate (RuBP). The enzyme which catalyzes this reaction (nicknamed RuBisCo) is the most abundant enzyme on earth! The resulting 6-carbon molecule is unstable, so it immediately splits into two 3-carbon molecules. The 3 carbons in the first stable molecule of this pathway give this largest group of plants the name “C-3.”
Dry air, hot temperatures, and bright sunlight slow the C-3 pathway for carbon fixation. This is because stomata, tiny openings under the leaf which normally allow CO2 to enter and O2 to leave, must close to prevent loss of water vapor (Figure 4.14). Closed stomata lead to a shortage of CO2. Two alternative pathways for carbon fixation demonstrate biochemical adaptations to differing environments.
www.ck12.org 234
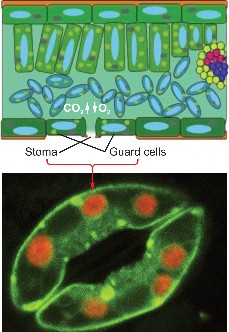
Plants such as corn solve the problem by using a separate compartment to fix CO2. Here CO2 combines with a 3-carbon molecule, resulting in a 4-carbon molecule. Because the first stable organic molecule has four carbons, this adaptation has the name C-4. Shuttled away from the initial fixation site, the 4-carbon molecule is actually broken back down into CO2, and when enough accumulates, Rubisco fixes it a second time! Compartmentalization allows efficient use of low concentrations of carbon dioxide in these specialized plants.
Cacti and succulents such as the jade plant avoid water loss by fixing CO2 only at night. These plants close their stomata during the day and open them only in the cooler and more humid nighttime hours. Leaf structure differs slightly from that of C-4 plants, but the fixation pathways are similar. The family of plants in which this pathway was discovered gives the pathway its name, Crassulacean Acid Metabolism, or CAM (Figure 4.15). All three carbon fixation pathways lead to the Calvin Cycle to build sugar.

As Melvin Calvin discovered, carbon fixation is the first step of a cycle. Like an electron transport chain, the Calvin cycle, shown in Figure 4.16, transfers energy in small, controlled steps. Each step pushes molecules uphill in terms of energy content. Recall that in the electron transfer chain, excited electrons lose energy to NADPH and ATP. In the Calvin Cycle, NADPH and ATP formed in the light reactions lose their stored chemical energy to build glucose.
Use the diagram below to identify the major aspects of the process:
the general cycle pattern
the major reactants
www.ck12.org 236
the products
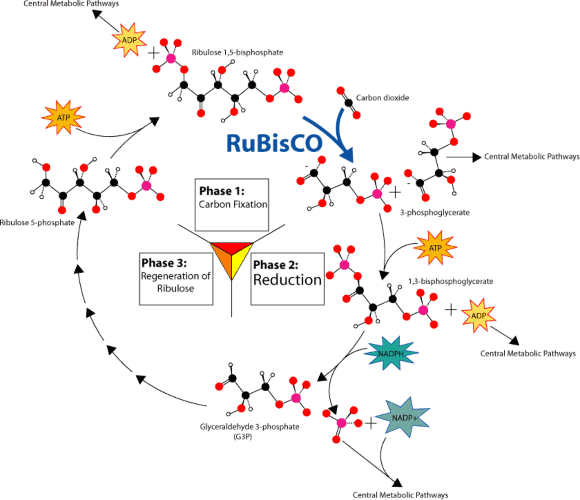
Figure 4.16: Overview of the Calvin Cycle Pathway. (13)
First, notice where carbon is fixed by the enzyme nicknamed Rubisco. In C-3, C-4, and CAM plants, CO2 enters the cycle by joining with 5-carbon ribulose bisphosphate to form a 6-carbon intermediate, which splits (so quickly that it isn’t even shown!) into two 3-carbon molecules.
Now look for the points at which ATP and NADPH (made in the light reactions) add chemical energy (“Reduction” in the diagram) to the 3-carbon molecules. The resulting “half-sugars” can enter several different metabolic pathways. One recreates the original 5- carbon precursor, completing the cycle. A second combines two of the 3-carbon molecules to form glucose, universal fuel for life.
The cycle begins and ends with the same molecule, but the process combines carbon and energy to build carbohydrates – food for life.
So – how does photosynthesis store energy in sugar? Six “turns” of the Calvin cycle use chemical energy from ATP to combine six carbon atoms from six CO2 molecules with 12 “hot hydrogens” from NADPH. The result is one molecule of glucose, C6H12O6.
The single chemical equation below represents the overall process of photosynthesis as well as summarizes many individual chemical reactions that were understood only after hundreds of years of scientific exploration.
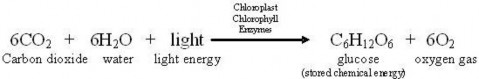
Chloroplasts are the organelles where the process of photosynthesis takes place in plants and algae.
Chloroplasts resemble blue green bacteria, containing their own DNA and ribosomes.
The Endosymbiotic Theory holds that chloroplasts once were independent prokary- otic cells, but were engulfed by other larger prokaryotes, forming the first eukaryotic cells.
Chloroplasts are made of membranes, which enclose stacks of membrane sacs called thylakoids.
The membranes sequence pigments and electron carrier molecules for efficient photosynthesis.
Thylakoids create compartments, which allow concentration gradients to store energy.
Pigment molecules absorb specific wavelengths (colors) of light; chlorophyll is the primary pigment in photosynthesis.
Electron carrier molecules form electron transport chains, which transfer energy in small steps so that the energy can be stored or used for work.
Photosynthesis consists of two groups of chemical reactions: the Light Reactions and the Calvin Cycle.
Light Reactions transform energy from sunlight into chemical energy, and produce and release oxygen gas.
When light strikes pigment molecules, electrons absorb its energy and are excited. www.ck12.org 238
Light also provides energy to split water molecules into electrons, hydrogen ions, and oxygen gas.
The oxygen gas is released as “waste”, but it is the source of the oxygen in Earth’s atmosphere.
Two pathways capture the energy from excited electrons as chemical energy stored in the bonds of molecules; both pathways involve electron transport chains.
One produces NADPH molecules, which stores energy and “hot hydrogen”.
A second pumps hydrogen ions into the thylakoids, forming an electrochemical gradient whose energy builds ATP molecules. This is “chemiosmosis”.
The Calvin Cycle uses the NADPH and ATP from the Light Reactions to “fix” carbon and produce glucose.
Stomata underneath plant leaves allow gases (CO2, H2O, and O2) to enter and exit the leaf interior.
Carbon dioxide enters the Calvin Cycle when an enzyme nicknamed “Rubisco” attaches it to a 5-carbon sugar. The unstable 6-carbon compound immediately breaks into two 3-carbon compounds, which continue the cycle.
Most plants fix CO2 directly with this pathway, so they are called C-3 plants.
Some plants have evolved preliminary fixation pathways, which help them conserve wa- ter in hot, dry habitats, but eventually the carbon enters the cycle along the “Rubisco” pathway.
C-4 plants such as corn use a 3-carbon carrier to compartmentalize initial carbon fixation in order to concentrate CO2 before sending it on to Rubisco.
CAM plants such as jade plants and some cacti open their stomata for preliminary CO2 fixation only at night.
In the Calvin Cycle, the fixed CO2 moves through a series of chemical reactions, gaining a small amount of energy (or “hot hydrogens”) from ATP or NADPH at each step.
Six turns of the cycle process 6 molecules of carbon dioxide and 12 “hot hydrogens” to produce a single molecule of glucose.
The cycle begins and ends with the same 5-carbon molecule, but the process stores chemical energy in food for nearly all life.
These interactive web sites depicts each step of photosynthesis in great detail.
http://www.johnkyrk.com/photosynthesis.html http://www.johnkyrk.com/photosynthesisdark.html
Summarize Jan Van Helmont’s willow tree experiment. State his conclusion and the in- ference he made after his experiment, and explain how his data supports each. Finally, relate his findings to what we know today about the overall process of photosynthesis.
Using the overall equation for photosynthesis, explain which components relate to J.B. Priestley’s observation that “Plants restore the air that animals injure.”
Explain how the structure of a chloroplast – its membranes and thylakoids – makes its function – the chemical reactions of photosynthesis – more efficient.
Summarize the Endosymbiotic Theory. What evidence related to chloroplasts supports this theory?
Name the two stages (sets of reactions) which make up the process of photosynthesis.
Match the major events with the stage of photosynthesis in which they occur. StagesLight Reactions
Calvin Cycle
Carbon dioxide is fixed.
Electrons in chlorophyll jump to higher energy levels.
Glucose is produced.
NADPH and ATP are produced.
NADPH and ATP are used.
Oxygen gas is released.
Water is split.
Use your understanding of pigments to explain why the living world appears green. Then think a little further and offer a hypothesis to explain why the world is not black!
Explain the value of cycles of chemical reactions, such as the Calvin Cycle.
Explain how their various methods of carbon fixation adapt C-3, C-4, and CAM plants to different habitats.
We humans depend on photosynthesis, and our actions in turn affect photosynthesis. Explain how humans depend on photosynthesis for:
food
building materials for furniture and homes
fuel for vehicles, heat, and electricity
breathable air
Explain how the following actions would affect photosynthesis:
We may clear-cut a forest for timber and parking lot space
When we burn fossil fuels for transportation or heat, we release CO2 into the atmosphere
When we dam up and overuse water in a certain area, the area water table drops www.ck12.org 240
Graham Kent, “Light Reactions in Photosynthesis” Animation. Bio 231 Cell Biology Lab, October 2004. Available on the web at:
http://www.science.smith.edu/departments/Biology/Bio231/ltrxn.html.
Illustrator: Thomas Porostocky; Writer: Lee Billings; Map data adapted from MODIS observations by NASA’s Terra and Aqua satellites; Graph data and reference: Biology, 4th ed., Neil A. Campbell, Benjamin/Cummings Publishing Company, 1996. “Crib Sheet #10, Photosynthesis.” Seed Magazine, August 2007. Available on the web at:
John Mynett, “Photosynthesis Animations.” Biology4All, 01 January 2002. Available on the web at:
http://www.biology4all.com/resources_library/details.asp?ResourceID=43
Kenneth R. Spring, Thomas J. Fellers, and Michael W. Davidson, “Introduction to Light and Energy.” Molecular Expressions Optical Microscopy Primer. The Physics of Light and Energy, Last modified Aug 23, 2005. Available on the web at:
http://micro.magnet.fsu.edu/primer/lightandcolor/lightandenergyintro.html.
Laurence A. Moran and Pearson Prentice Hall, “Fixing Carbon: The Rubisco reaction,” 10 July 2007. Sandwalk: Strolling with a Skeptical Biochemist,
http://sandwalk.blogspot.com/2007/07/fixing-carbon-rubisco-reaction.html.
“Photosynthesis”, “Electron Transport Chain” and “ATP Synthase” Animations. Vir- tual Cell Animation Collection, Molecular and Cellular Biology Learning Center, no date given. Available on the web at:
http://vcell.ndsu.nodak.edu/animations/photosynthesis/index.htm.
accessory pigment A molecule which absorbs colors of light other than blue-violet and red, and then transfers the energy to chlorophyll.
ATP synthase Ion channel and enzyme complex that chemically bonds a phosphate group to ADP, making ATP as H+ ions flow through the ion channel.
Calvin Cycle The second stage of photosynthesis, which can proceed without light, so its steps are sometimes called “light-independent” or “dark” reactions; results in the formation of a sugar.
carbon fixation The process which converts carbon dioxide in the air to organic molecules, as in photosynthesis.
chlorophyll The primary pigment of photosynthesis.
chloroplast The organelle in plant and algal cells where photosynthesis takes place.
electron carrier A molecule which transfers energy-carrying electrons within an electron transport chain.
electron transport chain (ETC) A series of electron-carrying molecules which accept and pass along energy-carrying electrons in small steps, allowing the energy lost at each transfer to be captured for storage or work.
endosymbiotic theory The theory which states that chloroplasts and mitochondria orig- inated as independent prokaryotic cells which were engulfed by larger prokaryotic cells to form the first eukaryotic cells.
glucose The carbohydrate product of photosynthesis; serves as the universal fuel for life.
light-dependent reactions The first set of reactions of photosynthesis; requires sunlight; also called the light reactions.
NADPH An energy carrier molecule produced in the light reactions of photosynthesis; used to build sugar in the Calvin cycle.
photolysis The light reaction process of splitting water molecules into electrons, hydrogen ions, and oxygen gas.
photosynthesis The process by which plants, algae, and some bacteria transform sunlight into chemical energy and use it to produce carbohydrate food and oxygen for almost all life.
photosystem A cluster of proteins and pigments found in chloroplasts and active in pho- tosynthesis.
pigment A molecule which absorbs specific wavelengths of light energy and reflects others and therefore appears colored.
RuBisCo The enzyme that combines one molecule of CO2 with a 5-carbon sugar called ribulose biphosphate (RuBP); the most abundant enzyme on earth.
stomata (singular stoma) Openings on the underside of a leaf which allow gas exchange and transpiration.
thylakoid Flattened sac-shaped compartment within a chloroplast, made of membranes embedded with molecules which carry out photosynthesis.
www.ck12.org 242
Recall Priestley’s early observation that plants “restore the air.” Name some ways that plants and algae affect the atmosphere.
Which of your own activities affect photosynthesis? Think “globally” in addition to “locally” and add large-scale human activities to your list. Are there any changes you could make in your life which could promote photosynthesis and a healthy atmosphere?
You learned in this chapter that plants make ”food” which life needs for energy. But is it usable energy? Or does it need to be converted into some other type of energy? What do you think and why?
http://commons.wikimedia.org/wiki/File: Chromatography_of_chlorophyll_-_Step_6.jpg. CC-BY-SA 2.5.
Alex Costa,Gross L, PLoS Biology Vol. 4/10/2006, ed. 358.
http://dx.doi.org/10.1371/journal.pbio.0040358. Public Domain,CC-BY.
Antje Boetius. http://biology.plosjournals.org/perlserv/?request= get-document&doi=10.1371/journal.pbio.0030102. CC-BY.
http://en.wikipedia.org/wiki/Image:Chlorophyll_structure.png. Public Domain.
http://www.flickr.com/photos/dropandroll/368377017. Creative Commons.
CK-12 Foundation. Joseph Priestly’s bell jar experiment.. CC-BY-SA.
http://www.flickr.com/photos/lobo235/76154752/ http://www.flickr.com/photos/jylcat/562393266/. CC-BY 2.0.
http://en.wikipedia.org/wiki/Image:Thylakoid_membrane.png. Public Domain.
http://commons.wikimedia.org/wiki/Image:Chloroplast-new.jpg. Public Domain.
http://en.wikipedia.org/wiki/Image:The_Earth_seen_from_Apollo_17.jpg. Public Domain.
Mike Jones. Overview of the Calvin Cycle Pathway.. CC-BY-SA 2.5.
http://commons.wikimedia.org/wiki/File:Kelp_300.jpg http:
//upload.wikimedia.org/wikipedia/commons/6/64/Anabaena_sperica.jpeg. (a)Public Domain (b)Public Domain (c)Creative Commons.
CK-12 Foundation. http://en.wikipedia.org/wiki/Image:Par_action_spectrum.gif. CC-BY-SA 2.0.
www.ck12.org 244
Clarify the relationship between breathing and cellular respiration.
Trace the flow of energy from food molecules through ATP to its use in cellular work.
Compare cellular respiration to burning.
Analyze the chemical equation for cellular respiration.
Briefly describe the role of mitochondria in producing ATP.
Compare cellular respiration to photosynthesis.
Show how carbon and oxygen atoms cycle through producers, consumers, and the environment.
Recognize that glycolysis is the first and most universal of three stages in cellular respiration.
Explain why biologists consider glycolysis to be one of the oldest energy production pathways.
Describe how some of the energy in glucose is transferred to ATP in the cytoplasm, without oxygen.
You know that humans deprived of oxygen for more than a few minutes will quickly become unconscious and die. Breathing, also known as respiration, is essential for human life, because the body cannot store oxygen for later use as it does food. The mammalian respiratory system, shown in Figure 5.1 features a diaphragm, trachea, and a thin membrane whose
surface area is equivalent to the size of a handball court - all for efficient oxygen intake. Other forms of life employ different types of respiratory organs: fish and aquatic amphibians and insects flaunt gills, spiders and scorpions develop ”book lungs,” and terrestrial insects use an elaborate network of tubes called tracheae, which open via spiracles, as shown in Figure 5.2 and Figure 5.3. A constant supply of oxygen gas is clearly important to life. However, do you know why you need oxygen?
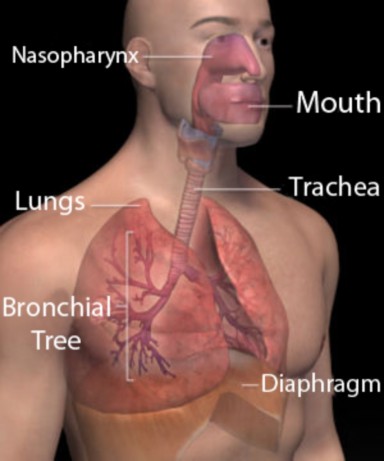
Many people would answer that oxygen is needed to make carbon dioxide, the gas exhaled or released by each of the respiratory systems listed above. However, CO2 is waste product. Surely, there is more to the story than just gas exchange with the environment! To begin to appreciate the role of oxygen inside your body, think about when your breathing rate increases: climbing a steep slope, running a race, or skating a shift in a hockey game. Respiration rate correlates with energy use, and that correlation reflects the link between oxygen and energy metabolism. For this reason, the chemical reactions inside your cells that
www.ck12.org 246

Figure 5.2: Spiracles in this Indian Luna Moth (Actias selene) caterpillar connect to a system of internal tubes (tracheae) which carry oxygen throughout the animal’s body. (20)

Figure 5.3: Gills in this alpine newt larva, Triturus alpestris, bring blood close to an extensive surface area so that the newt can absorb dissolved oxygen gas from its watery habitat. (15)
consume oxygen to produce usable energy are known as cellular respiration. This chapter will introduce you to the overall process of cellular respiration, and then focus on the first stage, which by itself does not require oxygen.
Another way to think about the role of oxygen in your body - and a good starting point for understanding the whole process of cellular respiration - is to recall the last time you sat by a campfire (see below figure) and noticed that it was ”dying.” Often people will blow on a campfire to keep it from ”dying out.” How does blowing help? What happens in a campfire?

You know that a fire produces light and heat energy. However, it cannot ”create” energy (remember that energy cannot be created or destroyed). Fire merely transforms the energy stored in its fuel – chemical energy – into light and heat. Another way to describe this energy transformation is to say that burning releases the energy stored in fuel. As energy is transformed, so are the compounds that make up the fuel. In other words, burning is a chemical reaction. We could write our understanding of this energy-releasing chemical reaction up to this point as:

www.ck12.org 248
Now return to what happens when you blow on a fire. The fire was ”dying out,” so you blew on it to get it going again. Was it movement or something in the air that promoted the chemical reaction? If you have ever ”smothered” a fire, you know that a fire needs something in the air to keep burning. That something turns out to be oxygen. Oxygen gas is a reactant in the burning process. At this point, our equation is:
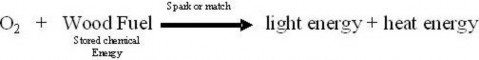
To complete this equation, we need to know what happens to matter, to the atoms of oxygen, and to the atoms of the fuel during the burning. If you collect the gas rising above a piece of burning wood in an inverted test tube, you will notice condensation - droplets appearing on the sides of the tube. Cobalt chloride paper will change from blue to pink, confirming that these droplets are water. If you add bromothymol blue (BTB) to a second tube of collected gases, the blue solution will change to green or yellow (Figure 5.5), indicating the presence of carbon dioxide. Thus, carbon dioxide and water are products of burning wood.

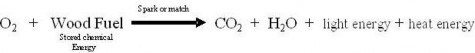
Now we know what happened to those oxygen atoms during the chemical reaction, but we need to be sure to identify the sources of the carbon atoms in the CO2 and of the hydrogen atoms in the water. If you guessed that these atoms make up the wood fuel – and nearly all fuels we burn, from coal to propane to candle wax to gasoline (hydrocarbons!), you have solved the equation completely. Overall, burning is the combining of oxygen with hydrogen and carbon atoms in a fuel (combustion or oxidation) to release the stored chemical energy as heat and light. Products of combustion are CO2 (oxidized carbon) and H2O (oxidized hydrogen). Or in symbols,
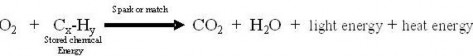
Return to the fate of the oxygen gas you breathe in and absorb. Recall that we related breathing rate and oxygen intake to energy use. Burning consumes oxygen as it releases stored chemical energy, transforming it into light and heat. Cellular respiration is actually a slow burn. Your cells absorb the oxygen carried by your blood from your lungs, and use the O2 to release stored chemical energy so that you can use it.
However, releasing energy within cells does not produce light or intense heat. Cells run on chemical energy – specifically, the small amount temporarily stored in adenine triphos- phate (ATP) molecules. Cellular respiration transfers chemical energy from a ”deliver- able” fuel molecule – glucose – to many ”usable” molecules of ATP. Like oxygen, glu- cose is delivered by your blood to your cells. If ATP were delivered to cells, more than 60,221,417,930,000,000,000,000,000 of these large molecules (which contain relatively small amounts of energy) would clog your capillaries each day. Pumping them across cell mem- branes would ”cost” a great deal of energy. A molecule of glucose contains a larger amount of chemical energy in a smaller package. Therefore, glucose is much more convenient for bloodstream delivery, but too ”powerful” to work within the cell. The process of cellular respiration uses oxygen to help transfer the chemical energy from glucose to ATP, which can be used to do work in the cell. This chemical equation expresses what we have worked out:
![]()
As with burning, we must trace what happens to atoms during cellular respiration. You can readily see that when the carbon atoms in glucose are combined with oxygen, they again form carbon dioxide. And when the hydrogen atoms in glucose are oxidized, they form water, as in burning. You can detect these products of cellular respiration in your breath on a cold day (as water condensation) and in the lab (BTB turns yellow when you blow into it through a straw). The equation:
www.ck12.org 250
![]()
This accounts for the energy transfer and the carbon, hydrogen, and oxygen atoms, but it does not show the ”raw materials” or reactants which build ATP. Recall that the energy temporarily stored in ATP is released for use when the bond between the second and third phosphates is broken. The resulting ADP can be recycled within the cell by recombining it with inorganic phosphate (Pi).
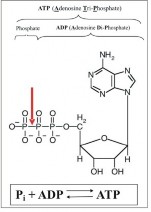
Now you should be able to see that the source of energy for re-attaching the phosphate is the chemical energy in glucose! Materials cycle and recycle, but energy gets used up and must be replaced. That is the key to understanding cellular respiration: it is a ”recharging of the batteries” - ATP molecules – which power cellular work. How many ATP can be made by harnessing the energy in a single glucose molecule? Although this number varies under certain conditions, most cells can capture enough energy from one molecule of glucose to build 38 molecules of ATP. Our equation becomes:
![]()
This equation for cellular respiration is not quite complete, however, because we can easily mix air and glucose sugar (even adding ADP and Pi) and nothing will happen. For the campfire, we indicated above the arrow that a necessary condition was a spark or match to start the reaction. A spark or match would damage or destroy living tissue. What necessary condition initiates the slow burn that is cellular respiration? Recall that enzymes are highly specific proteins which ”speed up” chemical reactions in living cells. More than 20 kinds of enzymes carry out cellular respiration! If you also recall that membranes within organelles often sequence enzymes for efficiency, as in chloroplasts for photosynthesis, you will not be surprised that a specific organelle, the mitochondrion (Figure 5.6), is also a necessary condition of cellular respiration - at least in eukaryotes.
www.ck12.org 252
Within each eukaryotic cell, the membranes of 1000-2000 mitochondria sequence enzymes and electron carriers and compartmentalize ions so that cellular respiration proceeds effi- ciently. Mitochondria, like chloroplasts, contain their own DNA and ribosomes and resem- ble certain bacteria. The endosymbiotic theory holds that mitochondria, too, were once independently living prokaryotes. Larger prokaryotes engulfed (or enslaved) these smaller aerobic cells, forming eukaryotic cells. Many prokaryotes today can perform cellular respira- tion; perhaps they and mitochondria have common ancestors. Their expertise in generating ATP made mitochondria highly valued symbionts.
Including these necessary conditions and balancing numbers of atoms on both sides of the arrow, our final equation for the overall process of cellular respiration is:
![]()
In words, cellular respiration uses oxygen gas to break apart the carbon-hydrogen bonds in glucose and release their energy to build 38 molecules of ATP. Most of this process occurs within the mitochondria of the cell. Carbon dioxide and water are waste products. This is similar to burning, in which oxygen breaks the carbon-hydrogen bonds in a fuel and releases their chemical energy as heat and light. Again, carbon dioxide and water are waste.
If you have studied the process of photosynthesis, you’ve probably already noticed its sim- ilarity to the process of cellular respiration. Both are processes within the cell which make chemical energy available for life. Photosynthesis transforms light energy into chemical en- ergy stored in glucose, and cellular respiration releases the energy from glucose to build ATP, which does the work of life. Moreover, photosynthesis reactants CO2 and H2O are products of cellular respiration. And the reactants of respiration, C6H12O6 and O2, are the products of photosynthesis. This interdependence is the basis of the carbon-oxygen cycle (Figure 5.7), which connects producers to consumers and their environment. At first glance, the cycle merely seems to show mitochondria undoing what chloroplasts do; but the cycle’s energy transformations power all the diversity, beauty, and mystery of life.
An excellent animation demonstrating cellular respiration can be found at the following web site:
http://videos.howstuffworks.com/hsw/10323-matter-and-energy-glycolysis-and-cellula htm
When was the last time you enjoyed yogurt on your breakfast cereal, or had a tetanus shot? These experiences may appear unconnected, but both relate to bacteria which do not use
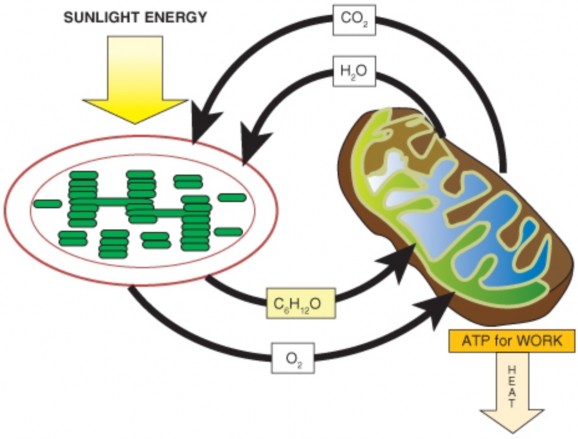
www.ck12.org 254
oxygen to make ATP. In fact, tetanus bacteria cannot survive if oxygen is present. However, Lactobacillus acidophilus (bacteria which make yogurt) and Clostridium tetani (bacteria which cause tetanus or lockjaw) share with nearly all organisms the first stage of cellular respiration, glycolysis (Figure 5.8). Because glycolysis is universal, whereas aerobic (oxygen- requiring) cellular respiration is not, most biologists consider it to be the most fundamental and primitive pathway for making ATP.
Figure 5.8: Clostridium tetani bacteria are obligate anaerobes, which cannot grow in the presence of oxygen and use a variation of glycolysis to make ATP. Because they can grow in deep puncture wounds and secrete a toxin, which can cause muscle spasms, seizures, and death, most people receive tetanus vaccinations at least every ten years throughout life. (9)
Return to the overall equation for cellular respiration:
![]()
Like photosynthesis, the process represented by this equation is actually many small, indi- vidual chemical reactions. We grouped the reactions of photosynthesis into two stages, the light reactions and the Calvin Cycle. We will divide the reactions of cellular respiration into three stages: glycolysis, the Krebs Cycle, and the electron transport chain (Figure 5.9). In this section, we will explore Stage 1, glycolysis - the oldest and most widespread pathway for making ATP. Before diving into the details, we must note that this first stage of cellular respiration is unique among the three stages: it does not require oxygen, and it does not take place in the mitochondrion. The chemical reactions of glycolysis occur without oxygen in the cytosol of the cell (Figure 5.10).
The name for Stage 1 clearly indicates what happens during that stage: glyco- refers to glucose, and -lysis means ”splitting.” In glycolysis, within the cytosol of the cell, a minimum of eight different enzymes break apart glucose into two 3-carbon molecules. The energy
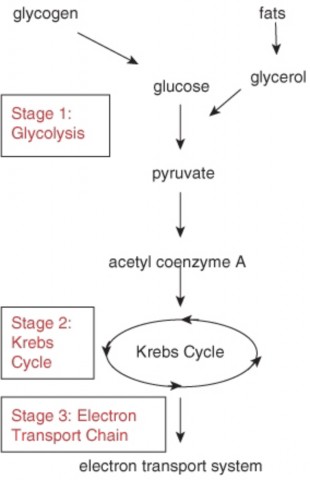
Figure 5.9: The many steps in the process of aerobic cellular respiration can be divided into three stages. The first stage, glycolysis, produces ATP without oxygen. Because this part of the cellular respiration pathway is universal, biologists consider it the oldest segment. Note that glycogen and fats can also enter the glycolysis pathway. (23)
www.ck12.org 256
released in breaking those bonds is transferred to carrier molecules, ATP and NADH. NADH temporarily holds small amounts of energy which can be used later to build ATP. The 3- carbon product of glycolysis is pyruvate, or pyruvic acid (Figure 5.11). Overall, glycolysis can be represented as shown below:
![]()
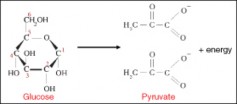
However, even this equation is deceiving. Just the splitting of glucose requires many steps, each transferring or capturing small amounts of energy. Individual steps appear in Figure
![]()
Studying the pathway in detail reveals that cells must ”spend” or ”invest” two ATP in order to begin the process of breaking glucose apart. Note that the phosphates produced by breaking apart ATP join with glucose, making it unstable and more likely to break apart. Later steps harness the energy released when glucose splits, and use it to build ”hot hydrogens” (NAD+ is reduced to NADH) and ATP (ADP + Pi ATP). If you count the
ATP produced, you will find a net yield of two ATP per glucose (4 produced – 2 spent). Remember to double the second set of reactions to account for the two 3-carbon molecules which follow that pathway! The ”hot hydrogens” can power other metabolic pathways, or in many organisms, provide energy for further ATP synthesis.
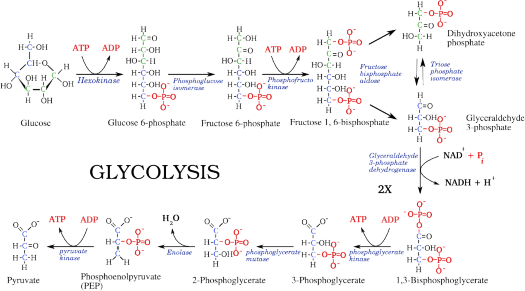
Figure 5.12: This detailed diagram demonstrates that glycolysis ”costs” 2 ATP, but har- nesses enough energy from breaking bonds in glucose to produce 4 ATP and 2 pairs of ”hot hydrogens” (NADH + H+). Note the multiplier (2X) required for the 3-carbon steps. (24)
To summarize: In the cytosol of the cell, glycolysis transfers some of the chemical energy stored in one molecule of glucose to two molecules of ATP and two NADH. This makes (some of) the energy in glucose, a universal fuel molecule for cells, available to use in cellular work
moving organelles, transporting molecules across membranes, or building large organic molecules.
Although glycolysis is universal, pathways leading away from glycolysis vary among species depending on the availability of oxygen. If oxygen is unavailable, pyruvate may be converted
www.ck12.org 258
to lactic acid or ethanol and carbon dioxide in order to regenerate NAD+, ending anaerobic respiration. Anaerobic respiration is also called fermentation, which we will discuss in a later section.
If oxygen is present, pyruvate enters the mitochondria for further breakdown, releasing far more energy and producing many more molecules of ATP in the latter two stages of aerobic respiration - the Krebs cycle and electron transport chain. We will explore these, too, in a later section.
Most organisms need oxygen for a single purpose: to release energy from food for use by cells.
Cellular respiration is a series of chemical reactions which transfer energy from glucose (deliverable or fuel energy) to ATP (usable energy).
Analyzing a campfire can clarify your understanding of cellular respiration. A campfire breaks chemical bonds in wood, releasing stored energy as light and heat; respiration breaks chemical bonds in glucose, releasing stored energy and transferring some to 38 ATP; some energy is lost as heat.
This equation summarizes the process of cellular respiration:
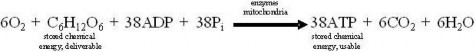
In eukaryotic cells, organelles called mitochondria sequence enzymes and electron car- riers and compartmentalize ions so that cellular respiration proceeds efficiently.
Cellular respiration, in many ways the opposite of photosynthesis, shows the interde- pendence of producers and consumers. Combined, the two equations demonstrate how energy flows and the carbon and oxygen cycle between organisms and environment.
The process of cellular respiration is actually many separate reactions, which can be divided into three stages: glycolysis, the Krebs Cycle, and the electron transport chain.
Why do nearly all organisms die without a constant supply of oxygen?
What source of energy do cells use to build ATP by cellular respiration?
Compare the purpose and energy content of glucose to the function and energy content of ATP; in other words, why do organisms need both kinds of energy-rich molecules?
Compare the process of burning gasoline in your automobile’s engine to the process of cellular respiration in terms of reactants, products, and necessary conditions.
Write out the chemical reaction which summarizes the overall process of cellular res- piration, first in symbols as a chemical equation, and then in words in a complete sentence.
In what eukaryote organelle does cellular respiration take place? Does this mean that prokaryotes cannot carry out the entire process of cellular respiration? Explain.
Compare and contrast cellular respiration and photosynthesis.
Diagram the carbon-oxygen cycle which connects producers, consumers, and their en- vironment. (P = producer, C = consumer).
List the three stages of cellular respiration, and contrast the first stage with the other two in terms of distribution throughout the living world, location within the cell, and use of oxygen.
Summarize the overall process of glycolysis, following both carbon atoms and chemical energy.
Martin Hoagland, Bert Dodson, and Judith Hauck, Exploring the Way Life Works: The Science of Biology. Jones and Bartlett Publishers, Inc., 2001. Chapter 3: ”Energy
Light to Life,” pp. 87-138.
Diana C. Linden and Roberta Pollack, ”Chart of Important metabolic products.” In Biology 130 Introduction to Cellular Biochemistry Lectures, Occidental College, last updated 21 October 2000. Available on the web at: http://departments.oxy.edu/ biology/bio130/lectures_2000/metabolic_products.htm
Environmental Protection Agency, ”Carbon Cycle Animation.” Climate Change, 23
October 2006. Available on the web at: http://epa.gov/climatechange/kids/ carbon_cycle_version2.html
John Kyrk, ”Glycolysis.” Cell Biology Animation, 28 April 2007. Available on the web
M.J. Farabee, 1992, 1994, 1997, 1999, 2000, 2001, 2007. ”Glycolysis, the Univer- sal Process.” Biobook, Estrella Mountain Community College, last modified 2007. Available on the web at: http://www.emc.maricopa.edu/faculty/farabee/BIOBK/ BioBookGlyc.html.
aerobic With oxygen, or living or occurring only in the presence of oxygen.
anaerobic Without oxygen; living or occurring in the absence of oxygen.
ATP Adenosine triphosphate; the universal energy “currency” for the cell; molecule which stores a usable amount of chemical energy.
www.ck12.org 260
cellular respiration The process which transfers chemical energy from glucose (a deliv- erable fuel molecule) to ATP (a usable energy-rich molecule).
cytosol The solution portion of a cell’s cytoplasm, consisting of water, organic molecules and ions.
endosymbiotic theory The theory which states that chloroplasts and mitochondria orig- inated as independent prokaryotic cells which were engulfed by larger prokaryotic cells to form the first eukaryotic cells.
glucose The carbohydrate product of photosynthesis; serves as the universal fuel for life.
glycogen Glucose molecules that have been chained together for energy storage; human muscle and liver cells store energy in this form.
glycolysis The process of splitting glucose” - stage 1 of aerobic cellular respiration and also the basis of anaerobic respiration; splits glucose into two 3-carbon pyruvates, producing 2 (net) ATP.
mitochondrion The “powerhouse” organelle in all eukaryotic cells where stages 2 (Krebs Cycle) and 3 (Electron Transport Chain) of aerobic respiration produce ATP.
NADH An electron carrier used to deliver energy to the electron transport chain of aerobic respiration.
symbiont An organism which lives in a close, mutually beneficial relationship with another organism.
In this lesson, you’ve learned that scientists consider glycolysis to be the oldest, or at least one of the oldest, pathways for making ATP. What might this say about earth’s ancient atmosphere? Can you imagine steps or events that might have been involved in the later evolution of aerobic cellular respiration, which includes glycolysis?
Prokaryotes can use either photosynthesis or cellular respiration – or both - to make ATP. Why do you think both processes evolved? Why not just photosynthesis? Which do you think came first in evolution? Why?
This lesson compares cellular respiration to burning. What activities in your daily life use burning? What are some consequences of those activities, in terms of materials produced and energy used?
Relate the history of oxygen in the atmosphere to the evolution of photosynthesis, aerobic respiration, mitochondria, and life on earth.
Describe the fate in eukaryotic cells of the pyruvate molecules produced by glycolysis if oxygen is present.
Recognize that for most organisms, if oxygen is present, the products of glycolysis enter the mitochondria for stage 2 of cellular respiration - the Krebs Cycle.
Trace carbon and hydrogen atoms through the Krebs Cycle.
Analyze the importance of the Krebs Cycle to cellular respiration by following the pathway taken by chemical energy.
Describe the structure of the mitochondrion, and identify the site of Krebs Cycle reactions.
Recognize that electron transport chain is the third and final stage of aerobic cellular respiration.
Describe how chemiosmotic gradients in mitochondria store energy to produce ATP.
Identify the role of oxygen in making stored chemical-bond energy available to cells.
Relate the structure of mitochondria to electron transport chain function and the production of ATP.
Enticing clues - volcanic gases, vast iron ore sediments, and bubbles of ancient air trapped in amber – suggest dramatic changes during the history of earth’s atmosphere. Correlating these clues with the fossil record leads to two major conclusions: that early life evolved in the absence of oxygen, and that oxygen first appeared between 2 and 3 billion years ago (Figure 5.13) because of photosynthesis by bluegreen bacteria (Figure 5.14). The chemistry of cellular respiration reflects this history. Its first stage, glycolysis, is universal and does not use oxygen.
Absolutely dependent on oxygen gas, we find it difficult to imagine that its appearance must have been disastrous for the anaerobic organisms that evolved in its absence. But oxygen is highly reactive, and at first, its effect on evolution was so negative that some have named this period the “oxygen catastrophe.” However, as oxygen gradually formed a protective ozone layer, life rebounded. After the first organisms “discovered” how to use oxygen to their advantage – in ways we will explore in this chapter – the diversity of aerobic organisms exploded. According to the endosymbiotic theory, engulfing of some of these aerobic bacteria led to eukaryotic cells with mitochondria, and multicellularity followed. Today, we
www.ck12.org 262
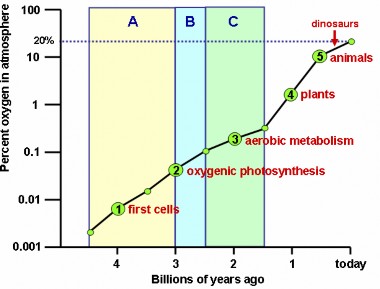

live in an atmosphere which is 21% oxygen, and most of life follows glycolysis with the last two, aerobic stages of cellular respiration.
Recall the purpose of cellular respiration: to release energy from glucose to make ATP - the universal “currency” for cellular work. The following equation describes the overall process, although it summarizes many individual chemical reactions.
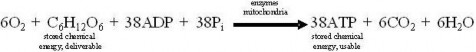
Once again, the first stage of this process, glycolysis, is ancient, universal, and anaerobic. In the cytoplasm of most cells, glycolysis breaks each 6-carbon molecule of glucose into two 3-carbon molecules of pyruvate. Chemical energy, which had been stored in the now broken bonds, is transferred to 2 ATP and 2 “hot hydrogens,” NADH.
The fate of pyruvate depends on the species and the presence or absence of oxygen. If oxygen is present to drive subsequent reactions, pruvate enters the mitochondrion, where the Krebs Cycle (Stage 2) and electron transport chain (Stage 3) break it down and oxidize it completely to CO2 and H2O. The energy thus released builds many more ATP molecules, though of course some is lost as heat. Let’s explore the details of how mitochondria use oxygen to make more ATP from glucose by aerobic respiration.
Aerobic respiration begins with the entry of pyruvate (product of glycolysis) into the mito- chondria. We will follow the six carbons of the original glucose molecule, so we will consider two 3-carbon pyruvates. The fate of pyruvate’s energy and carbon atoms can be followed in the examples below:
1. Within the mitochondria, each pyruvate is broken apart and combined with a coenzyme known as CoA to form a 2-carbon molecule, Acetyl CoA, which can enter the Krebs Cycle. A single atom of carbon (per pyruvate) is “lost” as carbon dioxide. The energy released in this breakdown is captured in two “hot hydrogen” – NADH. See Figure
5.15. Fatty acids can also break down into Acetyl CoA. By this means, lipids, like carbohydrates, can be “burned” to make ATP using the Krebs Cycle.
The Krebs Cycle (Figure 5.16) begins by combining each Acetyl CoA with a four- carbon carrier molecule to make a 6-carbon molecule of citric acid (or citrate, its ionized form). For this reason, the Krebs Cycle, named for a scientist who worked out its details, is also called the Citric Acid Cycle.
The cycle carries citric acid through a series of chemical reactions which gradually release energy and capture it in several carrier molecules. For each Acetyl CoA which enters the cycle, 3 NAD+ are reduced to NADH, one molecule of FAD (yet another tem-
www.ck12.org 264
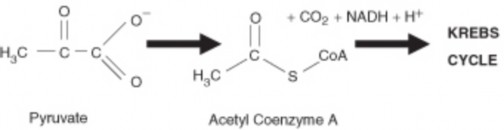
porary energy carrier we haven’t met before) is reduced to FADH2, and one molecule of ATP (actually a precursor, GTP) is made. Study Figure 5.16 to locate each of these energy-capturing events.
Note what happens to carbon atoms (black dots in Figure 5.16). For each 2-carbon Acetyl CoA which enters the cycle, two molecules of carbon dioxide are released - com- plete breakdown of the original 6-carbon glucose molecule. The final step regenerates the original 4-carbon molecule which began the cycle, so that another Acetyl CoA can enter.
In summary, the Krebs Cycle completes the breakdown of glucose which began with glycol- ysis. Its chemical reactions oxidize all six of the original carbon atoms to CO2, and capture the energy released in 2 ATP, 6 NADH, and 2 FADH2. These energy carriers join the 2 ATP and 2 NADH produced in glycolysis and the 2 NADH produced in the conversion of 2 pyruvates to 2 Acetyl CoA.
At the conclusion of the Krebs Cycle, glucose is completely broken down, yet only four ATP have been produced. Moreover, although oxygen is required to drive the Krebs Cycle, the cycle’s chemical reactions do not themselves consume O2. The conclusion of cellular respiration – its “grand finale!” – produces the majority of the ATP. The next section will explore the electron transport chain, where Stage 3 concludes aerobic cellular respiration.
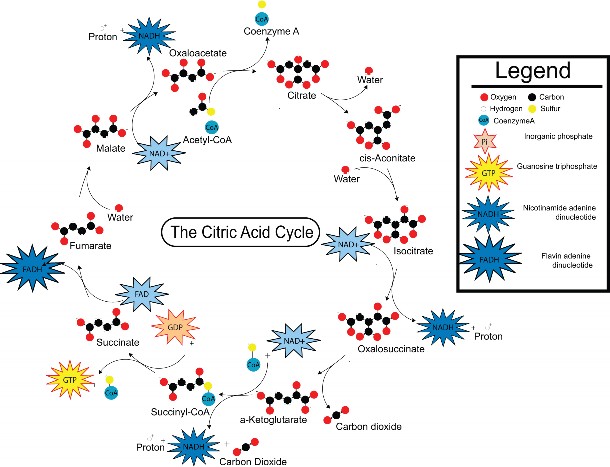
Figure 5.16: The Krebs or Citric Acid Cycle completes the breakdown of glucose begun in glycolysis. If oxygen is present, pyruvate enters the mitochondria and is converted to Acetyl CoA. Acetyl CoA enters the cycle by combining with 4-carbon oxaloacetate. Study the diagram to confirm that each turn of the cycle (two for each glucose) stores energy in 3 NADH+H+, one FADH2, and one ATP (from GTP), and releases 2 CO2. (14)
www.ck12.org 266
As noted earlier, the aerobic phases of cellular respiration in eukaryotes occur within or- ganelles called mitochondria. A detailed look at the structure of the mitochondrion (Figure 5.17) helps to explain its role in the last stage of respiration, the electron transport chain.
Two separate membranes form the mitochondrion. The inner membrane folds into cristae which divide the organelle into three compartments – intermembrane space (between outer and inner membranes), cristae space (formed by infoldings of the inner membrane), and matrix (within the inner membrane). The Krebs Cycle takes place within the matrix. The compartments are critical for the electron transport chain, as we’ll see in the final section of this lesson. Glycolysis occurs in the cytoplasm of the cell, with the products of glycolysis entering the mitochondria to continue cellular respiration.
At the end of the Krebs Cycle, energy from the chemical bonds of glucose is stored in diverse energy carrier molecules: four ATP, but also two FADH2 and ten NADH. The primary task of the last stage of cellular respiration, the electron transport chain (ETC), is to transfer energy from these carriers to ATP, the “batteries” which power work within the cell.
Pathways for making ATP in stage 3 of aerobic respiration closely resemble the electron transport chains used in photosynthesis. In both ETCs, energy carrier molecules are ar- ranged in sequence within a membrane so that energy-carrying electrons cascade from one to another, losing a little energy in each step. In both photosynthesis and aerobic respira- tion, the energy lost is harnessed to pump hydrogen ions into a compartment, creating an electrochemical or chemiosmotic gradient across the enclosing membrane. And in both processes, the energy stored in the chemiosmotic gradient is used to build ATP.
For aerobic respiration, the electron transport chain or “respiratory chain” is embedded in the inner membrane of the mitochondria (Figure 5.18). FADH2 and NADH (produced in glycolysis and the Krebs Cycle) donate high-energy electrons to energy carrier molecules within the membrane. As they pass from one carrier to another, the energy they lose is used to pump hydrogen ions into the intermembrane space, creating an electrochemical gradient. Hydrogen ions flow “down” the gradient – from outer to inner compartment – through an ion channel/enzyme, ATP synthase, which transfer their energy to ATP. Note the paradox that it requires energy to create and maintain a concentration gradient of hydrogen ions that are then used by ATP synthase to create stored energy (ATP). In broad terms, it takes energy to make energy. Coupling the electron transport chain to ATP synthesis with a hydrogen ion gradient is chemiosmosis, first described by Nobel laureate Peter D. Mitchell.
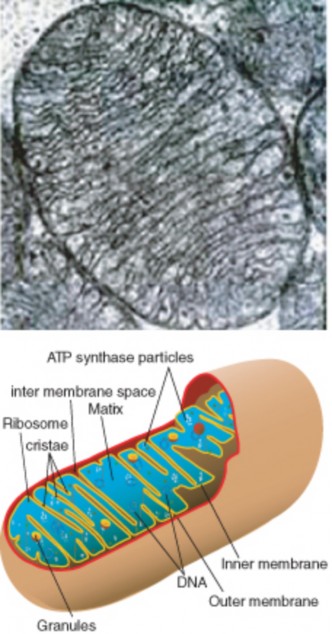
Figure 5.17: Mitochondria, organelles specialized to carry out aerobic respiration, contain an inner membrane folded into cristae, which form two separate kinds of compartments: inner membrane space and matrix. The Krebs Cycle takes place in the matrix. The electron transport chain is embedded in the inner membrane and uses both compartments to make ATP by chemiosmosis. (6)
www.ck12.org 268
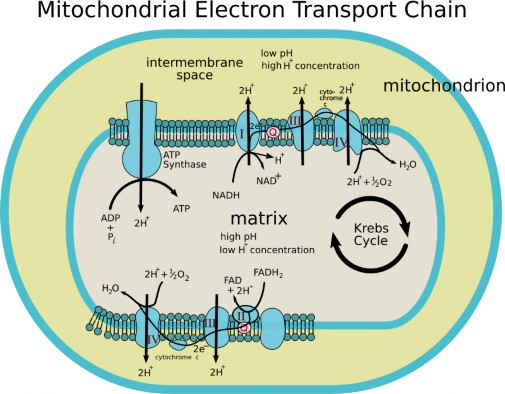
After passing through the ETC, low-energy electrons and low-energy hydrogen ions combine with oxygen to form water. Thus, oxygen’s role is to drive the entire set of ATP-producing reactions within the mitochondrion by accepting “spent” hydrogens. Oxygen is the final electron acceptor; no part of the process - from the Krebs Cycle through electron transport chain – can happen without oxygen.
The electron transport chain can convert the energy from one glucose molecule’s worth of FADH2 and NADH+H+ into as many as 34 ATP. When the four ATP produced in glycolysis and the Krebs Cycle are added, the total fits the overall equation for aerobic cellular respiration:
![]()
Aerobic respiration is complete. If oxygen is available, cellular respiration transfers the energy from one molecule of glucose to 38 molecules of ATP, releasing carbon dioxide and water as waste. “Deliverable” food energy has become energy which can be used for work within the cell – transport within the cell, pumping ions and molecules across membranes, and building large organic molecules. Can you see how this could lead to “life in the fast lane” compared to anaerobic respiration (glycolysis alone)?
Introduction to Aerobic Respiration:
Oxygen produced by the first photosynthetic organisms was probably toxic to the anaerobic life forms which then populated the earth, but later organisms evolved a way to harness the power of oxygen to make ATP. This new pathway was aerobic respiration.
In eukaryotic cells, if oxygen is present, the pyruvate molecules produced by glycolysis in the cytoplasm enter the mitochondria for further breakdown and energy release.
The Krebs Cycle harnesses the energy which remains in pyruvate after glycolysis.
For most organisms, if oxygen is present, the products of glycolysis enter the mito- chondria for stage 2 of cellular respiration - the Krebs cycle.
In the mitochondrion, 3-carbon pyruvate combines with Coenzyme A to form 2-carbon Acetyl CoA and CO2, storing released energy in NADH.
Acetyl CoA enters the Krebs Cycle by combining with a 4-carbon molecule to form citric acid.
The Krebs Cycle removes energy from citric acid in small steps, storing it in diverse energy carrier molecules: ATP, NADH and FADH2.
www.ck12.org 270
The Krebs Cycle produces two molecules of CO2 per Acetyl CoA, completing the breakdown of glucose.
Mitochondria are organelles whose membranes are specialized for aerobic respiration.
The matrix of the mitochondria is the site of Krebs Cycle reactions.
The electron transport chain and most ATP synthesis rely on the compartments created by the inner membrane of the mitochondria.
The third and final stage of aerobic cellular respiration, the electron transport chain, accounts for most of the ATP.
Stage 3 transfers the energy from NADH and FADH2 to make ATP.
High-energy electrons from these two energy carriers pass along electron acceptors embedded in the inner membrane of the mitochondria.
As the electrons flow, the electron acceptors capture small amounts of energy to pump hydrogen ions out into the intermembrane space.
These concentrated hydrogen ions store potential energy as an electrochemical gradient.
Hydrogen ions flow back into the inner membrane space through channel proteins, which use their energy to build ATP. This is chemiosmosis.
The ETC coupled with the hydrogen ion flow can build 34 ATP per glucose molecule.
When ATP from glycolysis and the Krebs Cycle are added, a total of 38 ATP result from aerobic respiration of one molecule of glucose.
Interactive animation depicting the steps of cellular respiration.
http://www.uwmc.uwc.edu/biology/respiration/cellresp.html
Animation detailing the steps of electron transport chain.
http://vcell.ndsu.edu/animations/etc/movie-flash.htm
Animation detailing the H+ concentration gradient and ATP Synthase.
http://vcell.ndsu.edu/animations/atpgradient/movie-flash.htm
Explain why the appearance of oxygen in the atmosphere between two and three billions of years ago was both “good news and bad news” for life on Earth.
In eukaryotic cells when oxygen is present, what is the fate of the pyruvate produced in glycolysis?
Trace the six carbon atoms originally from glucose through the Krebs Cycle.
Trace the flow of energy from the pyruvates produced in glycolysis through the Krebs Cycle.
Describe the structure of the mitochondrion, and identify the sites of the Krebs Cycle and the Electron Transport Chain.
Summarize the overall task of Stage 3 of aerobic respiration.
List the steps in stage 3 which produce ATP.
Name the three stages of aerobic cellular respiration. Then write the overall equation, and identify which stage:
uses each reactant
requires each necessary condition and
produces each product.
Explain the principle of chemiosmosis.
Predict the main idea of the next lesson by comparing the energy available to anaerobic organisms, which use just glycolysis to make ATP, to the energy available to aerobic organisms, which use all three stages of cellular respiration to make ATP.
Martin Hoagland, Bert Dodson, and Judith Hauck, Exploring the Way Life Works: The Science of Biology. ones and Bartlett Publishers, Inc., 2001. Chapter 3: “Energy
– Light to Life,” pp. 87-138.
Diana C. Linden and Roberta Pollack, “Chart of Important metabolic products.” In Biology 130 Introduction to Cellular Biochemistry Lectures, Occidental College, last updated 21 October 2000. Available on the web at: http://departments.oxy.edu/ biology/bio130/lectures_2000/metabolic_products.htm
“Electron Transport Chain, The Movie.” Virtual Cell Animation Collection, Molecular
and Cellular Biology Learning Center, 1998-2006. Available on the web at: http:
Graham Kent, “An animation of the Tricarboxylic Acid Cycle.” Biology 231 Cell Biol- ogy Laboratory, October 2004. Available on the web at: http://www.science.smith. edu/departments/Biology/Bio231/krebs.html.
Graham Kent, “Electron Transport Chain.” Biology 231 Cell Biology Laboratory, Oc-
tober 2004. Available on the web at: http://www.science.smith.edu/departments/ Biology/Bio231/etc.html.
www.ck12.org 272
OSU Marching Band, “Ohio State University presents the Krebs Cycle,” You Tube, 9 October 2006. (http://www.youtube.com/watch?v=FgXnH087JIk.)
John Kyrk, “Animated Krebs Cycle.” Cell Biology Animation, 12 April 2007. Available
John Kyrk, “Animated essentials of mitochondria and the electron transport chain.” Cell Biology Animation, 12 April 2007. Available on the web at: http://www.johnkyrk. com/mitochondrion.html.
Gabe Simon & Dr. Jeff Brodsky, “Citric Acid Cycle.” Bioscience 1820 Interactive
Pathways Study Guide, 2003.
http://www.pitt.edu/AFShome/j/b/jbrodsky/public/html/1820/tca.htm
ATP Adenosine triphosphate; the universal energy “currency” for the cell; molecule which stores a usable amount of chemical energy.
ATP synthase Ion channel and enzyme complex that chemically bonds a phosphate group to ADP, making ATP as H+ ions flow through the ion channel.
chemiosmosis Process in cellular respiration or photosynthesis which produces ATP using the energy of hydrogen ions diffusing from high concentration to low.
chemiosmotic gradient In cellular respiration or photosynthesis, a difference in concen- tration of hydrogen ions across a membrane within the mitochondrion or chloroplast set up using energy from an electron transport chain.
cristae The space formed by infoldings of the inner membrane within the mitochondrian.
electrochemical gradient A difference in both electrical charge and chemical concentra- tion across a membrane.
electron transport chain (ETC) A series of electron-carrying molecules which accept and pass along energy-carrying electrons in small steps, allowing the energy lost at each transfer to be captured for storage or work.
endosymbiotic theory The theory which states that chloroplasts and mitochondria orig- inated as independent prokaryotic cells which were engulfed by larger prokaryotic cells to form the first eukaryotic cells.
FADH2 An electron carrier used to deliver energy to the electron transport chain of aerobic respiration.
glycolysis The process of “splitting glucose” - stage 1 of aerobic cellular respiration and also the basis of anaerobic respiration; splits glucose into two 3-carbon pyruvates, producing 2 (net) ATP.
Krebs Cycle Stage 2 of aerobic cellular respiration; a series of chemical reactions which completes the breakdown of glucose begun in stage 1, releasing more chemical energy and producing carbon dioxide; also called the Citric Acid Cycle.
intermembrane space The space between the outer and inner membranes of the mito- chondrian.
matrix The space within the inner membrane of the mitochondrian.
mitochondrion The “powerhouse” organelle in all eukaryotic cells where stages 2 (Krebs Cycle) and 3 (Electron Transport Chain) of aerobic respiration produce ATP.
NADH An electron carrier used to deliver energy to the electron transport chain of aerobic respiration.
According to the endosymbiotic theory, although some prokaryotes evolved aerobic respiration, eukaryotes took the short-cut of engulfing these prokaryotes rather than “re-inventing the wheel.” The benefits to the “host” cells are obvious. What might have been some of the benefits to the prokaryote?
Cycles, electron transport chains, and chemiosmosis are common to both photosynthe- sis and cellular respiration. Why do you think they’re found in both energy pathways?
Distinguish between obligate aerobes, obligate anaerobes, and facultative anaerobes.
Explain that, in the absence of oxygen fermentation reactions must regenerate NAD+ in order for glycolysis to continue making ATP.
Discuss how your muscles continue to work for you even when your respiratory and cardiovascular system can no longer keep up a continuous supply of oxygen.
www.ck12.org 274
Identify yourself as “sprinter” or “endurance runner” and predict the type of muscle fiber (red or white) which predominates in your body.
Describe how bacteria, including those we employ to make yogurt, make ATP in the absence of oxygen.
Compare and contrast alcoholic and lactic acid fermentation pathways.
Outline the process used to produce fuel from corn.
Explain how we employ anaerobic organisms to make bread, beer, and wine.
Compare the energy efficiency of aerobic cellular respiration to that of fermentation.
List the advantages of anaerobic over aerobic respiration.
Explain why vertebrate muscles use both aerobic and anaerobic pathways to make ATP.
After the photosynthetic “oxygen catastrophe” challenged life between 2.5 and 3 billion years ago, evolution rebounded with biochemical pathways to harness and protect against oxygen’s power. Today, most organisms use O2 in aerobic respiration to produce ATP. Almost all animals, most fungi, and some bacteria are obligate aerobes, which require oxygen. Some plants and fungi and many bacteria retain the ability to make ATP without oxygen. These facultative anaerobes use ancient anaerobic pathways when oxygen is limited. A few bacteria remain as obligate anaerobes, which die in the presence of oxygen and depend on only the first (anaerobic) stage of cellular respiration.
Aerobic and anaerobic pathways diverge after glycolysis splits glucose into two molecules of pyruvate:
![]()
Pyruvate still contains a great deal of chemical energy. If oxygen is present, pyruvate enters the mitochondria for complete breakdown by the Krebs Cycle and electron transport chain. If oxygen is not present, cells must transform pyruvate to regenerate NAD+ in order to continue making ATP. Two different pathways accomplish this with rather famous products: lactic acid and ethyl alcohol (Figure 5.19). Making ATP in the absence of oxygen by glycolysis alone is known as fermentation. Therefore, these two pathways are called lactic acid fermentation and alcoholic fermentation. If you lack interest in organisms, such as yeast and bacteria, which have “stuck with” the anaerobic tradition, the products of these chemical reactions may still intrigue you. Fermentation makes bread, yogurt, beer, wine, and some new biofuels. In addition, some of your body’s cells are facultative anaerobes, retaining one of these ancient pathways for short-term, emergency use.
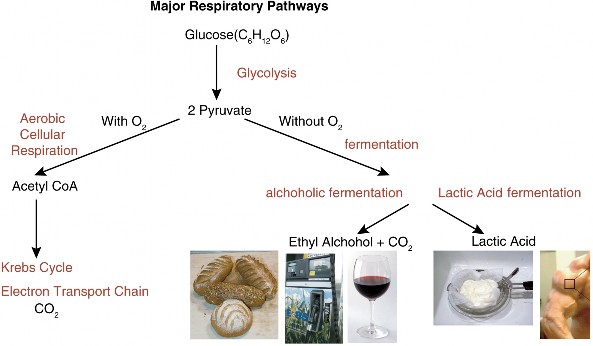
www.ck12.org 276
For chicken or turkey dinners, do you prefer light meat or dark? Do you consider yourself a sprinter, or a distance runner? (Figure 5.20)
Yes! Muscle color reflects its specialization for aerobic or anaerobic metabolism. Although humans are obligate aerobes, our muscle cells have not given up on ancient pathways which allow them to keep producing ATP quickly when oxygen runs low. The difference is more pronounced in chickens and grouse (Figure 5.21), which stand around all day on their legs. For long periods of time, they carry out aerobic respiration in their “specialized- for-endurance” red muscles. If you have ever hunted grouse, you know that these birds “flush” with great speed over short distances. Such “sprinting” flight depends on anaerobic respiration in the white cells of breast and wing muscle. No human muscle is all red or all white, but chances are, if you excel at running short distances or at weight lifting, you have more white glycolytic fibers in your leg muscles. If you run marathons, you probably have more red oxidative fibers.
You probably were not aware that muscle cells “ferment.” Lactic acid fermentation is the type of anaerobic respiration carried out by yogurt bacteria (Lactobacillus and others) and by your own muscle cells when you work them hard and fast. Converting pyruvate to 3-carbon lactic acid (see Figure below) regenerates NAD+ so that glycolysis can continue to make ATP in low-oxygen conditions.

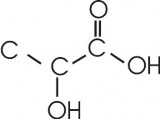
![]()
For Lactobacillusbacteria, the acid resulting from fermentation kills bacterial competitors in buttermilk, yogurt, and some cottage cheese. The benefits extend to humans who enjoy these foods, as well (Figure 5.22).
You may have noticed this type of fermentation in your own muscles, because muscle fatigue and pain are associated with lactic acid. Keep this in mind, however, as we discuss a second type of fermentation, which produces alcohol. Imagine what would happen as you ran a race if muscle cells conducted alcoholic rather than lactic acid fermentation!
Have you fueled your car with corn? You have, if you bought gas within the city of Portland, Oregon. Portland was the first city to require that all gasoline sold within the city limits
www.ck12.org 278

Figure 5.22: Lactobacillus bacteria use the same type of anaerobic respiration as our muscle cells. Lactic acid reduces competition from other bacteria, and flavors yogurt, as well! (4)
contain at least 10% ethanol. By mid-2006, nearly 6 million “flex-fuel” vehicles – which can use gasoline blends up to 85% ethanol (E85 – Figure 5.23) were traveling US roads. This “new” industry employs an “old” crew of yeast and bacteria to make ethanol by an even older biochemical pathway – alcoholic fermentation. Many people consider “renewable” biofuels such as ethanol a partial solution to the declining availability of “nonrenewable” fossil fuels. Although controversy still surrounds the true efficiency of producing fuel from corn, ethanol is creeping into the world fuel resource picture (Figure 5.24).

You are probably most familiar with the term ”fermentation” in terms of alcoholic beverages. You may not have considered that the process is actually a chemical reaction certain bacteria and yeasts use to make ATP. Like lactic acid fermentation, alcoholic fermentation processes pyruvate one step further in order to regenerate NAD+ so that glycolysis can continue to make ATP. In this form of anaerobic respiration, pyruvate is broken down into ethyl alcohol and carbon dioxide:
![]()
We have domesticated yeast (Figures 5.25 and Figure 5.26) to carry out this type of anaerobic respiration for many commercial purposes. When you make bread, you employ the yeast to make the bread “rise” by producing bubbles of carbon dioxide gas. Why do you suppose that eating bread does not intoxicate you?
Brewers of beer and wine use yeast to add alcohol to beverages. Traditional varieties of yeast not only make but also limit the quantity of alcohol in these beverages, because above 18% by volume, alcohol becomes toxic to the yeast itself! We have recently developed new strains of yeast which can tolerate up to 25% alcohol by volume. These are used primarily in the production of ethanol fuel.
Human use of alcoholic fermentation depends on the chemical energy remaining in pyruvate after glycolysis. Transforming pyruvate does not add ATP to that produced in glycolysis, and for anaerobic organisms, this is the end of the ATP-producing line. All types of anaerobic respiration yield only 2 ATP per glucose. In the next section, we will compare the advantages and disadvantages of aerobic and anaerobic respiration.
As aerobes in a world of aerobic organisms, we tend to consider aerobic respiration “better” than fermentation. In some ways, it is. However, anaerobic respiration has persisted far longer on this planet, through major changes in atmosphere and life. There must be value
www.ck12.org 280

in this alternative way of making ATP. In this last section, we will compare the advantages and disadvantages of these two types of respiration.
A major argument in favor of aerobic over anaerobic respiration is overall energy production. Without oxygen, organisms can only break 6-carbon glucose into two 3-carbon molecules. As we saw earlier, glycolysis releases only enough energy to produce two (net) ATP per molecule of glucose. In contrast, aerobic respiration breaks glucose all the way down to CO2, producing up to 38 ATP. Membrane transport costs can reduce this theoretical yield, but aerobic respiration consistently produces at least 15 times as much ATP as anaerobic respiration. This vast increase in energy production probably explains why aerobic organisms have come to dominate life on earth. It may also explain how organisms were able to increase in size, adding multicellularity and great diversity.
However, anaerobic pathways persist, and a few obligate anaerobes have survived over 2 billion years beyond the evolution of aerobic respiration. What are the advantages of fer- mentation?
One advantage is available to organisms occupying the few anoxic (lacking oxygen) niches remaining on earth. Oxygen remains the highly reactive, toxic gas which caused the “Oxygen Catastrophe.” Aerobic organisms have merely learned a few tricks – enzymes and antioxidants
to protect themselves. Organisms living in anoxic niches do not run the risk of oxygen exposure, so they do not need to spend energy to build these elaborate chemicals.
Individual cells which experience anoxic conditions face greater challenges. We mentioned earlier that muscle cells “still remember” anaerobic respiration, using lactic acid fermentation to make ATP in low-oxygen conditions. Brain cells do not “remember”, and consequently cannot make any ATP without oxygen. This explains why death follows for most humans who endure more than four minutes without oxygen.
Variation in muscle cells gives further insight into some benefits of anaerobic respiration. In vertebrate muscles, lactic acid fermentation allows muscles to produce ATP quickly during short bursts of strenuous activity. Muscle cells specialized for this type of activity show differences in structure as well as chemistry. Red muscle fibers are “dark” because they have a rich blood supply for a steady supply of oxygen, and a protein, myoglobin, which holds extra oxygen. They also contain more mitochondria, the organelle in which the Krebs cycle and electron transport chain conclude aerobic respiration. White muscle cells are “light” because they lack the rich blood supply, have fewer mitochondria, and store glycogen rather than oxygen. When you eat dark meat, you are eating endurance muscle. When you eat white meat, you are eating muscle built for sprinting.
Each type of muscle fiber has advantages and disadvantages, which reflect their differing biochemical pathways. Aerobic respiration in red muscles produces a great deal of ATP from far less glucose - but slowly, over a long time. Anaerobic respiration in white muscles produces ATP rapidly for quick bursts of speed, but a predator who continues pursuit may eventually catch a white-muscled prey.
www.ck12.org 282
In summary, aerobic and anaerobic respiration each have advantages under specific condi- tions. Aerobic respiration produces far more ATP, but risks exposure to oxygen toxicity. Anaerobic respiration is less energy-efficient, but allows survival in habitats which lack oxy- gen. Within the human body, both are important to muscle function. Muscle cells specialized for aerobic respiration provide endurance, and those specialized for lactic acid fermentation support short but intense energy expenditures. Both ways of making ATP play critical roles in life on earth.
In the two to three billion years since photosynthesis added oxygen to earth’s atmo- sphere, life has become mostly aerobic. Some organisms and types of cells retain the older, anaerobic pathways for making ATP; these pathways comprise anaerobic respi- ration or fermentation.
Obligate aerobes require oxygen to make ATP. Obligate anaerobes cannot survive in the presence of oxygen, so they occupy only anoxic habitats. Facultative anaerobes make ATP with oxygen, but if oxygen levels become low, they can use fermentation.
Some bacteria, including those we employ to make yogurt, make ATP using lactic acid fermentation; the acid may help reduce competition from other bacteria. Muscle cells can continue to produce ATP when O2 runs low using lactic acid fermentation, but muscle fatigue and pain may result.
Red muscle fibers use mostly aerobic respiration to make ATP for endurance tasks; white muscle fibers use mostly lactic acid fermentation to make ATP quickly for short, intense activities. Human muscles contain a mixture of red and white fibers, but genetics may give sprinters more white fibers, and marathoners more red.
Both alcoholic and lactic acid fermentation pathways change pyruvate in order to continue producing ATP by glycolysis.
Ethanol produced by bacteria through alcoholic fermentation of corn (and perhaps other fuels in the near future) may provide a more renewable fuel for vehicles than the fossil fuels upon which we currently depend. We employ yeasts to help make bread through alcoholic fermentation; as they produce carbon dioxide, the bread dough rises. We employ anaerobic organisms to make beer and wine through alcoholic fermentation; the alcohol content is limited to 18% by volume because levels above that are toxic to these organisms.
Aerobic respiration is far more energy-efficient than anaerobic respiration. Aerobic processes produce up to 38 ATP per glucose. Anaerobic processes yield only 2 ATP per glucose.
Classify your own cells as obligate aerobes, obligate anaerobes, or facultative anaerobes, and explain your reasoning. (Although these terms usually apply to whole organisms, assume they can also apply to individual cells within your body).
Identify yourself as a “sprinter” or an “endurance runner” and predict the type of muscle fiber (red or white) which predominates in your body. Explain your reasoning.
Construct a chart which compares alcoholic to lactic acid fermentation, considering at least three different features.
Outline the process used to produce fuel from corn and explain why some consider this fuel “renewable” and preferable to fossil fuels. Research the pros and cons of this fuel.
Explain how fermentation is used to make bread.
If two species of bacteria – one using aerobic respiration and the other using anaerobic respiration - were competing for the same source of glucose in the same environment, which one would out-compete the other? Explain why.
Human cells cannot carry out alcoholic fermentation, yet we use it for many purposes. Analyze its importance to human life.
Explain why both types of fermentation must change pyruvic acid, even though no energy is gained in this conversion.
Indicate the maximum alcohol content of wine and beer, and explain the reason for this limit.
Construct a chart comparing aerobic to anaerobic respiration using at least 5 charac- teristics.
Martin Hoagland, Bert Dodson, and Judith Hauck, Exploring the Way Life Works: The Science of Biology. Jones and Bartlett Publishers, Inc., 2001. Chapter 3: “Energy
Light to Life,” pp. 87-138.
“Biology, Answering the Big Questions of Life/Fermentation student lab” and “Biol- ogy, Answering the Big Questions of Life/Fermentation.“ Wikibooks, last modified 13 March 2007. Available on the web at: http://en.wikibooks.org/wiki/Biology%2C_ Answering_the_Big_Questions_of_Life/Fermentation
Diana C. Linden and Roberta Pollack, “Chart of Important metabolic products.” In
Biology 130 Introduction to Cellular Biochemistry Lectures, Occidental College, last updated 21 October 2000. Available on the web at:
http://departments.oxy.edu/biology/bio130/lectures_2000/metabolic_products. htm
J. Stein Carter, 1996, “Cellular Respiration and Fermentation.” last modified on Tue
02 Nov 2004. Available on the web at:
John Kyrk, “Glycolysis.” Cell Biology Animation, 28 April 2007. Available on the web www.ck12.org 284
M.J. Farabee, 1992, 1994, 1997, 1999, 2000, 2001, 2007. “Glycolysis, the Univer- sal Process.” Biobook, Estrella Mountain Community College, last modified 2007. Available on the web at: http://www.emc.maricopa.edu/faculty/farabee/BIOBK/ BioBookGlyc.html
aerobic With oxygen, or living or occurring only in the presence of oxygen.
alcoholic fermentation The process for making ATP in the absence of oxygen, by con- verting glucose to ethanol and carbon dioxide.
anaerobic Without oxygen; living or occurring in the absence of oxygen.
facultative anaerobe An organism which can respire aerobically when oxygen is present, but is also capable of fermentation when oxygen levels are low.
glycolysis The process of “splitting glucose” - stage 1 of aerobic cellular respiration and also the basis of anaerobic respiration; splits glucose into two 3-carbon pyruvates, producing 2 (net) ATP.
lactic acid fermentation The process for making ATP in the absence of oxygen by con- verting glucose to lactic acid.
obligate aerobe An organism which requires oxygen for cellular respiration.
obligate anaerobe An organism which uses anaerobic respiration, and dies in the presence of oxygen.
Humans seem to harness anaerobic respiration much more than aerobic respiration to create useful products, such as foods or fuels. Use your understanding of the two processes to explain why this makes sense.
Some controversy exists over whether or not ethanol produced by fermentation of corn is an efficient and wise way to produce fuel. Can you think of some reasons, pro and/or con?
How might the wing muscles of birds which migrate long distances compare to those of birds which do not migrate? Why do you suppose human muscles are mixtures of red and white fibers, rather than specialized, as in many birds?
Christian Fischer. http://commons.wikimedia.org/wiki/Image:CyanobacteriaInPool.jpg. CC-BY-SA 3.0.
CK-12 Foundation. http://pam.wikipedia.org/wiki/File:3DScience_respiratory_labeled.jpg. CC-BY-SA.
http://www.flickr.com/photos/oskarn/459851554/. Mikol Graves,CC-BY-SA 2.0.
http://www.flickr.com/photos/momthebarbarian/2441500/. CC-BY. (5) http://www.flickr.com/photos/65189390@N00/622469923/. CC-BY.
http://commons.wikimedia.org/wiki/Image: Diagram_of_a_human_mitochondrion.svg. Public Domain.
CK-12 Foundation,MesserWoland and Szczepan.
http://commons.wikimedia.org/wiki/Image:Biological_cell.svg. GNU-FDL.
http://commons.wikimedia.org/wiki/Image:Acetyl-CoA.svg. GNU-FDL.
CDC. http://commons.wikimedia.org/wiki/Image:Clostridium_tetani_01.png. Public Domain.
http://commons.wikimedia.org/wiki/Image:Alpha-d-glucose.png. GNU-FDL.
CK-12 Foundation. http://www.flickr.com/photos/kosherpickle/201168636/ http://commons.wikimedia.org/wiki/Image:Red_Wine_Glas.jpg http://www.flickr.com/photos/momthebarbarian/2441500/ http://commons.wikimedia.org/wiki/Image:Skeletal_muscle_diagram.jpg. CC-BY-SA 2.0,CC-BY-SA 2.5,CC-BY 2.0,GNU-FDL.
http://commons.wikimedia.org/wiki/Image:Oxygen_atmosphere.png. Public Domain.
http://commons.wikimedia.org/wiki/Image:MitoChondria.jpg. Public Domain.
http://en.wikipedia.org/wiki/Image:TCA.svg. CC-BY-SA 2.5. www.ck12.org 286
André Karwath. http://commons.wikimedia.org/wiki/Image:Smooth_Newt_larva_%28aka%29.jpg. CC-BY-SA 2.5.
Túrelio (http://commons.wikimedia.org/wiki/User:T%C3%BArelio). http://commons.wikimedia.org/wiki/Image:HolzfeuerFlussbett.jpg. CC-BY-SA 2.5.
http://commons.wikimedia.org/wiki/Image:Yeast_cell_english.svg. (a)CC-BY-SA 2.5,(b)GNU-FDL.
Brandon Fesser. http://commons.wikimedia.org/wiki/Image:Bromothymol_blue_colors.jpg. Public Domain.
CK-12 Foundation. http://commons.wikimedia.org/wiki/File:Chloroplast.png. Public Domain,GNU-FDL.
Shawn Hanrahan. http://commons.wikimedia.org/wiki/Image: Actias_selene_5th_instar_spiracles_sjh.jpg. CC-BY-SA 2.5.
http://commons.wikimedia.org/wiki/Image: Mitochondrial_electron_transport_chain.png. CC-BY-SA 2.5.
http://www.flickr.com/photos/kosherpickle/201168636/. CC-BY 2.0,CC-BY-SA.
CK-12 Foundation. http://commons.wikimedia.org/wiki/Image: Cellular_respiration_flowchart_%28en%29.svg. Public Domain.
http://commons.wikimedia.org/wiki/Image:GlycolysiscompleteLabelled.png. Public Domain.
http://sca21.wikia.com/wiki/File:World_renewable_energy_2005.png. Public Domain.
http://commons.wikimedia.org/wiki/Image:Red_Wine_Glas.jpg. (a)GNU-FDL,(b)CC-BY-SA.
www.ck12.org 288
Describe the properties of cell division in prokaryotes.
Describe cell division in eukaryotes. Explain the main differences between cell division in prokaryotic and eukaryotic cells.
Describe the basic properties of chromosomes.
Describe the key steps in the cell cycle.
Identify and describe the main processes in mitosis.
Describe how the cell cycle is controlled and define cancer.
You are made of many different types of cells. Nerve cells, skin cells, muscle cells, and many more. These cells obviously have many different functions, yet they all develop from the first cell that makes you. So do they all have the same DNA? Are all the cells in your body genetically identical? How does the first cell of an organism know to become two cells, then four cells, and so on? What tells these cells what to do? Your body produces about 25 million genetically identical cells every second. These new cells are formed when older cells divide, a process called cell division or cell reproduction.
Cell division is the final step in the life of a cell, otherwise known as the cell cycle. Eukaryotic cells and prokaryotic cells complete this process by a number of different mechanisms. The cell cycle is a repeating series of events, during which the eukaryotic cell carries out its necessary functions, including metabolism, cellular growth, and division, resulting in two genetically identical daughter cells. To produce two genetically identical daughter cells, the
chromosomes need to replicate and the nucleus and cytoplasm need to divide. These are key events in the life of a cell.
Prokaryotic organisms reproduce asexually by binary fission, a process that produces iden- tical offspring (Figure 6.1). In asexual reproduction, a single parent produces genetically identical offspring. As prokaryotes do not have a nucleus, and have only one circular chromo- some, they do not need to reproduce by the same mechanism as eukaryotic cells. Prokaryotic cell division is a much simpler process. In prokaryotic cell division, after the single chromo- some is copied, the cell grows larger. Eventually the two chromosomes separate and move to opposite ends of the cell. Newly formed cell membrane then grows into the center of the cell, separating the two chromosomes, and forming two genetically identical daughter cells. The formation of two daughter cells is called cytokinesis.
Under ideal conditions, reproduction in bacteria is extremely efficient, with some bacteria reproducing every 20 minutes. This makes bacteria an extremely effective tool for the molec- ular biologist. However, bacteria do not usually live in ideal conditions; otherwise, bacteria would grow and divide extremely rapidly, eventually covering the surface of Earth. Bacterial growth is limited by nutrients and water, predation, and by their own wastes.
Cell division in eukaryotic organisms is very different from that in prokaryotes, mainly be- cause of the many chromosomes in the nuclei of eukaryotic cells. Cell division in eukaryotic organisms is necessary for development, growth, and repair. This cell division ensures that each resulting daughter cell receives a complete copy of the organism’s entire genome. Re- member that all of an organism’s DNA must be present in each somatic, or body, cell. This DNA contains the information necessary for that cell to perform its functions, and to give that organism its traits. Therefore, prior to cell division, the eukaryotic cell’s complete genome must be copied, ensuring that each daughter cell receives a complete set of the genome.
The formation of gametes, an organism’s reproductive cells, such as sperm and egg cells, involves a completely different method of cell division. This cell division ensures that each gamete receives half the amount of an organism’s DNA.
As previously discussed (in the Foundations of Life Science chapter), DNA contains the information necessary to make proteins, direct a cell’s activities, and give an organism its traits. This information is organized into structural units scattered along the length of the
www.ck12.org 290
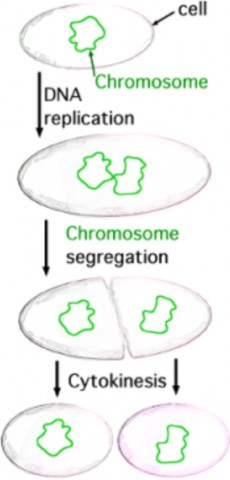
DNA molecule. These units are known as genes. A gene contains the information necessary to encode an RNA molecule or a protein. A single DNA molecule contains hundreds to thousands of genes. Different cell types use the information in different genes to make different proteins. This process gives different cell types distinct activities. Thus, a liver cell will have many different proteins than a kidney cell, giving the two cells types distinct activities. When a cell is using the information within a gene, the segment of DNA containing that gene is unwound, exposing the double helix to the cell machinery needed to use that information.
Prior to cell division, the DNA must duplicate itself in a process called DNA replication. This ensures that each resulting cell receives a complete set of the organism’s genome. But how is the replicated DNA divided up evenly? What guarantees that each new cell will receive a complete set of DNA? It was the identification of chromosomes that allowed this process to be characterized. As a eukaryotic cell prepares to divide, the DNA and associated proteins (histones) coil into a structure, known as a chromosome (Figure 6.2). The DNA copies itself prior to this process, so the chromosome that forms consists of two identical chromatids, known as sister chromatids, identical copies of DNA. The two chromatids are attached at a region called the centromere. The chromatids separate from each other when the nucleus divides just prior to cell division. Thus, each new cell that results after cell division will have the complete amount of genetic material, identical to the original, or parent, cell. In human cells, this amounts to 46 chromosomes. These chromosomes come in pairs (one from each pair inherited from each parent). So these 46 chromosomes are actually two sets of 23 chromosomes each. For an animation of how the DNA coils into a chromosome, see http://www.hhmi.org/biointeractive/media/DNAi_packaging_vo2-sm.mov.
Each human somatic cell (a body cell, or every cell other than a gamete) normally has two sets of chromosomes, one set inherited from each parent. Each set contains 23 chromosomes, for a total of 46 chromosomes. Each chromosome differs in size, from over 250 million nucleotide pairs to less than 50 million nucleotide pairs. Each chromosome contains a specific set of genes, making each chromosome essential to survival.
Each pair of chromosomes consists of two chromosomes that are similar in size, shape, and genes. These pairs of chromosomes are known as homologous chromosomes, or homologues. Upon fertilization, a zygote is formed (Figure 6.3). A zygote is the first cell of a new individual. In humans, a zygote contains 23 pairs (or two sets) of chromosomes. Any cell containing two sets of chromosomes is said to be diploid. The zygote forms from the fusion of two haploid gametes. A haploid cell contains one set of chromosomes. In humans, a haploid gamete contains 23 chromosomes. Biologists use the symbol n to represent one set of chromosomes, and 2n to represent two sets. In humans, each set of chromosomes contains 22 autosomes and 1 sex chromosome. Autosomes are chromosomes that are not directly involved in determining the sex of an individual. The sex chromosomes contain genes that determine the sex of an individual.
Whereas autosomes are found as homologous pairs in somatic cells, sex chromosomes come in two different sizes, shapes, and contain different genes. In many organisms, including
www.ck12.org 292
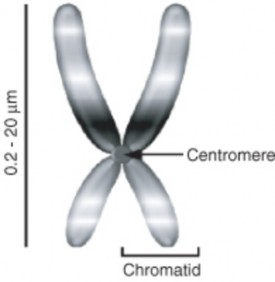
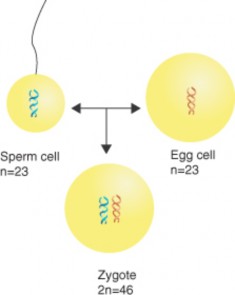
humans, the sex chromosomes are known as the X and Y chromosomes. The Y chromosome contains genes that cause male development. Therefore, any individual with a Y chromosome is male, and a male will have both an X and Y chromosome (XY). Females, without a Y chromosome, will have two X chromosomes (XX). As females have two X chromosomes, they must pass an X chromosome to all of their children. As males have both an X chromosome (inherited from their mother) and a Y chromosome, they can give either chromosome to their children. If a child inherits a Y from his father, he will be male; if a child inherits an X from her father, she will be female. It therefore is the male gamete that determines the sex of the offspring.
Cell division in eukaryotic cells is much more complex than in prokaryotic cells because of the many chromosomes within the nucleus. Both the cytoplasm and the genetic material must be divided, ensuring that each resulting daughter cell receives 46 separate chromosomes. To ensure this, in addition to the cell performing its necessary functions, the DNA must be copied, as must many organelles, prior to cell division.
The life of a eukaryotic cell is a cycle, known as the cell cycle (Figure 6.4). The cell cycle is a repeating series of cellular growth and division. The cell cycle has five phases: the first growth (G1) phase, the synthesis (S) phase, the second growth (G2) phase, mitosis, and
www.ck12.org 294
cytokinesis, though many consider mitosis and cytokinesis to be combined into one phase. The cell spends the majority of the cycle in the first three phases of the cycle, collectively known as interphase. After cytokinesis, two genetically identical daughter cells are formed.
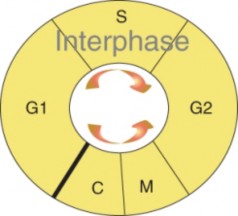
Figure 6.4: The Cell Cycle. The cell cycle depicts the life of an eukaryotic cell. The cell cycle has five phases: the first growth (G1) phase, the synthesis (S) phase, the second growth (G2) phase, mitosis (M), and cytokinesis (C). The cell spends the majority of the cycle in the first three phases (G1, S, G2) of the cycle, collectively known as interphase. After cytokinesis, two genetically identical daughter cells are formed. Many consider the cell cycle to only have four phases, with mitosis and cytokinesis combined. http://www.cellsalive.com/cell_ cycle.htm has an excellent animation of the cell cycle. (3)
The first growth (G1) phase: The cell spends most of its life in the G1 phase. During this phase, a cell undergoes rapid growth and the cell performs its routine functions. If a cell is not dividing, the cell remains in this phase.
The synthesis (S) phase: For two genetically identical daughter cells to be formed, the cell’s DNA must be copied or replicated. When the DNA is replicated, both strands of the double helix are used as templates to produce two new complementary strands. These new strands then hydrogen bond to the template strands and two double helices form.
The second growth (G2) phase is a shortened growth period in which many organelles are reproduced or manufactured. Parts necessary for cell division are made during G2.
Mitosis is the phase of nuclear division, in which one nucleus divides and becomes two nuclei. After mitosis is cytokinesis, in which the cytoplasm divides in half, producing two daughter cells, each containing a complete set of genetic material.
Mitosis is the division of the cell’s nucleus, the final step before two daughter cells are produced. The cell enters mitosis as it approaches its size limitations. Four distinct phases
of mitosis have been recognized: prophase, metaphase, anaphase, and telophase, with each phase merging into the next one (Figure 6.5).
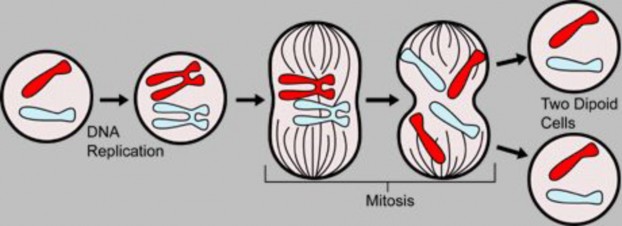
Figure 6.5: During mitosis, the nucleus divides, paving the way for two cells to be produced after cell division, each with a complete makeup of genetic material. http://www.biology. arizona.edu/Cell_bio/tutorials/cell_cycle/MitosisFlash.html has an excellent an- imation of mitosis. (1)
Prophase is the first and longest phase of mitosis. During prophase, the DNA coils up into visible chromosomes, each made up of two sister chromatids held together by the centromere. The nucleus disappears as the nuclear envelope and nucleolus break apart. The centrioles begin to move to opposite ends, or poles, of the cell. As the centrioles migrate, the fiber-like spindle begins to elongate between the centrioles. The spindle is a thin, cage-like structure made out of microtubules. In plant cells, the spindle forms without centrioles. The spindle plays an essential role moving chromosomes and in the separation of sister chromatids.
During metaphase the spindle attaches to the centromere of each chromosome. Helped by the spindle, the chromosomes line up at the center, or equator, of the cell, also known as the metaphase plate. Each sister chromatid is attached to a separate spindle fiber, with one fiber extending to one pole, and the other fiber extending to the other pole. This ensures that the sister chromatids separate and end up in distinct cells after cell division.
Anaphase is the phase in which the sister chromatids separate. The sister chromatids are pulled apart by the shortening of the microtubules of the spindles, similar to the reeling in of a fish by the shortening of the fishing line. One sister chromatid moves to one pole of the cell, and the other sister chromatid moves to the opposite pole. At the end of anaphase, each pole of the cell has a complete set of chromosomes, identical to the amount of DNA at the beginning of G1 of the cell cycle.
Telophase is essentially the opposite of prophase. The chromosomes begin to unwind in preparation to direct the cell’s metabolic activities. The spindle begins to break down, allowing a new nucleus to form. This is followed by cytokinesis, the division of the cytoplasm,
www.ck12.org 296
resulting in two genetically identical cells, ready to enter G1 of the next cell cycle. The phases of mitosis are summarized in Figure 6.6.
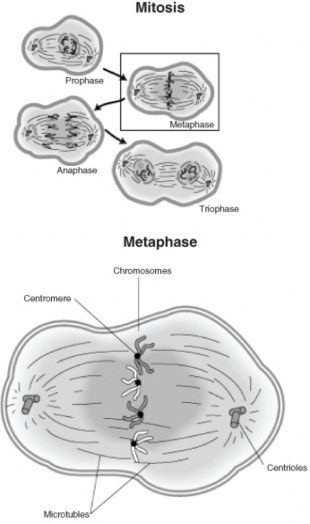
Cytokinesis (Figure 6.7) differs between plant and animal cells. In animal cells, the plasma membrane pinches inward along the cell’s equator until two cells are formed. In plant cells, a cell plate forms along the cells equator. A new membrane grows along each side of the cell plate, with a new cell wall forming on the outside of each new membrane.
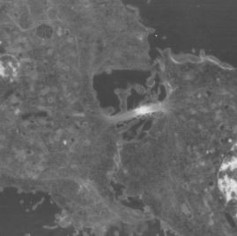
How does the cell know when to divide? How does the cell know when to replicate the DNA? The answers to these questions have to do with the control of the cell cycle. But how is the cell cycle controlled?
The cell cycle is controlled by a number of protein-controlled feedback processes. Two types of proteins involved in the control of the cell cycle are kinases and cyclins. Cyclins activate kinases. Cyclins are a group of proteins that is rapidly produced at key stages in the cell cycle. Kinases activate other target molecules. It is this precise regulation of proteins that triggers advancement through the cell cycle.
The cell cycle has key checkpoints. When the cell receives key signals or information (feed- back regulation), the cell can begin the next phase of the cell cycle. The cell can also receive signals that delay passage to the next phase of the cell cycle. These signals allow the cell to complete the previous phase before moving forward. Three key checkpoints are the cell growth (G1) checkpoint, the DNA synthesis (G2) checkpoint, and the mitosis checkpoint.
The cell growth (G1) checkpoint allows the cell to proceed into the S phase of the cell cycle and continue on to divide. The cell spends most of the cycle in the G1 phase. G1 is where the cell carries out its main functions. If the cell has performed its functions and has grown to significant size to be divided in half, key proteins will stimulate DNA replication to begin. If the cells are not to divide, such as some muscle and nerve cells, the cell will stop at this checkpoint and move into a resting phase. Some cells may stay in this resting period permanently, never dividing.
The DNA synthesis (G2) checkpoint determines if the cell is ready for mitosis. DNA repair enzymes check the replicated DNA at this point. If the checkpoint is passed, the many
www.ck12.org 298
molecular mechanisms and processes needed for mitosis will begin.
The mitosis checkpoint determines the end of one cycle and the beginning of the next. This checkpoint signals the end of mitosis, allowing the cell to prepare for the beginning of G1 of the next cell cycle.
Many cancers result from uncontrolled cell division, when the regulation of the cycle is lost (Figure 6.8). Cancerous cells divide much more rapidly than healthy cells. These cells use the blood and nutrients that other cells need and they can stress the environment of the healthy cells. As cancerous cells do not provide any useful function to the organism, they are extremely harmful. If cancerous cells are allowed to grow uncontrolled, they will kill the host organism. Many cancerous cells are the products of normal cells that have lost the ability to regulate the cell cycle. The genes that encode the proteins involved in cell cycle regulation have mutations. One category of genes, called oncogenes, accelerate the cell cycle. Many cancers can be inherited, such as breast cancer. Others are triggered by an environmental stimulus, such as through the relationship between tobacco smoke and lung cancer, or ultraviolet radiation and skin cancer.
The cell cycle is a repeating series of events, characterizing the life of a eukaryotic cell.
Binary fission is a form of cell division in prokaryotic organisms that produces identical offspring.
As a eukaryotic cell prepares to divide, the DNA and associated proteins coil into a structure, known as a chromosome.
The DNA copies during the S phase of the cell cycle, resulting in a chromosome that consists of two identical chromatids, known as sister chromatids, attached at a region called the centromere.
Any cell containing two sets of chromosomes is said to be diploid; the zygote forms from the fusion of two haploid gametes.
The cell cycle has five phases: the first growth (G1) phase, the synthesis (S) phase, the second growth (G2) phase, mitosis, and cytokinesis.
Mitosis is the division of the nucleus; four distinct phases of mitosis have been recog- nized: prophase, metaphase, anaphase, and telophase.
Cytokinesis is the division of the cytoplasm.
The cell cycle is controlled through feedback mechanisms.
Cancer results from uncontrolled cell division, due to the loss of regulation of the cell cycle.
www.ck12.org 300
How does cell division in bacteria differ from mitosis in eukaryotes?
Describe the structure of a chromosome in prophase of mitosis.
What is cytokinesis and when does it occur?
What is a centromere?
Describe interphase.
Describe the main steps of mitosis.
What is binary fission?
Define a gene.
http://nobelprize.org/educational_games/medicine/2001
autosomes Chromosomes that are not directly involved in determining the sex of an in- dividual.
binary fission Asexual reproduction in prokaryotic organisms; produces identical offspring.
cancer Disease that can result from uncontrolled cell division, when the regulation of the cycle is lost.
cell cycle A repeating series of events, during which the eukaryotic cell carries out its necessary functions, including metabolism, cellular growth, and division, resulting in two genetically identical daughter cells.
cell division Process of cell formation from the division of older cells.
cell plate Forms during cytokinesis in plant cells; a new membrane grows along each side of the cell plate, with a new cell wall forming on the outside of each new membrane.
centriole Structure from which spindle fibers originate.
centromere Region that attaches two sister chromatids; approximately near the middle of a chromosome.
chromosome Coiled structure of DNA and histone proteins; allows for the precise sepa- ration of replicated DNA; forms during prophase of mitosis and meiosis.
cyclins A group of proteins that is rapidly produced at key stages in the cell cycle; activate kinases; participate in the regulation of the cell cycle.
cytokinesis Division of the cytoplasm, forming two daughter cells.
diploid A cell containing two sets of chromosomes; in human cells, two sets contains 46 chromosomes.
DNA replication Process by which the DNA is copied, resulting in two identical copies.
gametes An organism’s reproductive cells, such as sperm and egg cells.
gene A segment of DNA that contains the information necessary to encode an RNA molecule or a protein.
haploid A cell containing one set of chromosomes; in human gametes, one set is 23 chro- mosomes.
homologous chromosomes A pair of chromosomes (one from each parent) consisting of two chromosomes that are similar in size, shape, and genes; also known as homologues.
interphase The first three phases of the cell cycle; the cell spends the majority of its time here.
kinases Proteins involved in the regulation of the cell cycle; activated by cyclins; activate other target molecules.
metaphase plate The center (equator) of a cell during mitosis; chromosomes line up at the metaphase plate to ensure the proper separation of the chromatids.
mitosis The division of the nucleus into two genetically identical nuclei.
oncogene Cancer causing gene; can accelerate the cell cycle. www.ck12.org 302
resting phase Phase associated with the G1 phase of the cell cycle; cells that do not divide are in a resting phase and do not continue to the S phase.
S phase Synthesis phase; the phase of the cell cycle in which the DNA is replicated (copied).
sex chromosomes Contain genes that determine the sex of an individual.
sister chromatid Identical copies of a DNA molecule; a chromosome at the start of mitosis and meiosis has two sister chromatids.
spindle Thin, cage-like fibers made out of microtubules; used to move chromosomes and to separate the sister chromatids during mitosis.
zygote The first cell of a new individual.
A human cell has 46 chromosomes, while a bacterial cell has only one chromosome. Would you think that the number of chromosomes relates to the complexity of the cell or organism?
Mitosis and cytokinesis produce two genetically identical daughter cells. Think about how a cell with half as much DNA, such as a sex cell, may form.
As not every species has members of the opposite sex, such as bacteria, yet all organisms must reproduce to stay alive, think about how these sexless organisms may reproduce.
Describe asexual reproduction; explain the genetic relationship between parent and offspring.
Describe sexual reproduction; explain the genetic relationship between parent and offspring.
Identify and describe the main steps of meiosis, distinguishing between the quantity of genetic material in the parent and resulting cells.
Describe gametogenesis and identify the key differences between oogenesis and sper- matogenesis.
Distinguish between the three types of sexual life cycles.
Some organisms look and act exactly like their parent. Others share many similar traits, but they are definitely unique individuals. Some species have two parents, whereas others have just one. How an organism reproduces determines the amount of similarity the organism will have to its parent. Asexual reproduction produces an identical individual, whereas sexual reproduction produces a similar, but unique, individual. In sexual reproduction, meiosis produces haploid gametes that fuse during fertilization to produce a diploid zygote (Figure
and Figure 6.11).
Are there male and female bacteria? How could you tell? Remember, bacteria have just one chromosome; they do not have an X or Y chromosome. So they probably have a very simplified form of reproduction. Asexual reproduction, the simplest and most primitive method of reproduction, produces a clone, an organism that is genetically identical to its parent. Haploid gametes are not involved in asexual reproduction. A parent passes all of its genetic material to the next generation. All prokaryotic and many eukaryotic organisms reproduce asexually.
There are a number of types of asexual reproduction including fission, fragmentation and budding. In fission, a parent separates into two or more individuals of about equal size. In fragmentation, the body breaks into several fragments, which later develop into complete adults. In budding, new individuals split off from existing ones. The bud may stay attached
www.ck12.org 304
or break free from the parent. Eukaryotic organisms, such as the single cell yeast and multicellular hydra, undergo budding (Figure 6.10).
Figure 6.10: Magnification of a budding yeast. (8)
Why do you look similar to your parents, but not identical? First, it is because you have two parents. Second, it is because of sexual reproduction.
Whereas asexual reproduction produces genetically identical clones, sexual reproduction pro- duces genetically diverse individuals. As both parents contribute half of the new organism’s genetic material, the offspring will have traits of both parents, but will not be exactly like either parent.
Organisms that reproduce sexually by joining gametes, a process known as fertilization, must have a mechanism to produce haploid gametes. This mechanism is meiosis, a type of cell division that halves the number of chromosomes. During meiosis the pairs of chromosomes separate and segregate randomly to produce gametes with one chromosome from each pair. Meiosis involves two nuclear and cell divisions without an interphase in between, starting with one diploid cell and generating four haploid cells (Figure 6.11). Each division, named meiosis I and meiosis II, has four stages: prophase, metaphase, anaphase, and telophase. These stages are similar to those of mitosis, but there are distinct and important differences.
Prior to meiosis, the cell’s DNA is replicated, generating chromosomes with two sister chro- matids. A human cell prior to meiosis will have 46 chromosomes, 22 pairs of homologous autosomes, and 1 pair of sex chromosomes. Homologous chromosomes are similar in size, shape, and genetic content. You inherit one chromosome of each pair from your mother and the other one from your father.
The 8 stages of meiosis are summarized below. The stages will be described for a human cell, starting with 46 chromosomes.
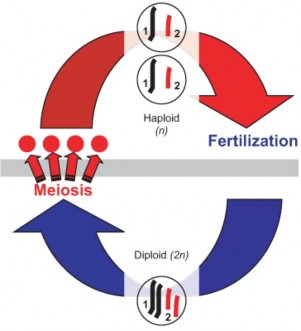
www.ck12.org 306
Prophase I: prophase I is very similar to prophase of mitosis, but with one very significant difference. In Prophase I, the nuclear envelope breaks down, the chromosomes condense, and the centrioles begin to migrate to opposite poles of the cell, with the spindle fibers growing between them. During this time, the homologous chromosomes form pairs. These homologous chromosomes line up gene-for-gene down their entire length, allowing crossing- over to occur. This is an important step in creating genetic variation and will be discussed later.
Metaphase I: In metaphase I, the 23 pairs of homologous chromosomes line up along the equator of the cell. During mitosis, 46 individual chromosomes line up during metaphase. Some chromosomes inherited from the father are facing one side of the cell, and some are facing the other side.
Anaphase I: During anaphase I the spindle fibers shorten, and the homologous chromosome pairs are separated from each other. One chromosome from each pair moves toward one pole, with the other moving toward the other pole, resulting in a cell with 23 chromosomes at one pole and the other 23 at the other pole. The sister chromatids remain attached at the centromere. Because human cells have 23 pairs of chromosomes, this independent assortment of chromosomes produces 223, or 8,388,608 possible configurations. More on independent assortment of chromosomes will be presented in the chapter on Mendelian Genetics.
Telophase I: The spindle fiber disassembles and the nucleus reforms. This is quickly followed by cytokinesis and the formation of two haploid cells, each with a unique combination of chromosomes, some from the father and the rest from the mother. After cytokinesis, both cells immediately enter meiosis II; the DNA is not copied in between. Meiosis II is essentially the same as mitosis, separating the sister chromatids from each other.
Prophase II: Once again the nucleus breaks down, and the spindle begins to reform as the centrioles move to opposite sides of the cell.
Metaphase II: The spindle fibers align the 23 chromosomes, each made out of two sister chromatids, along the equator of the cell.
Anaphase II: The sister chromatids are separated and move to opposite poles of the cell. As the chromatids separate, each is known as a chromosome. Anaphase II results in a cell with 23 chromosomes at each end of the cell; each chromosome contains half as much genetic material as at the start of anaphase II.
Telophase II: The nucleus reforms and the spindle fibers break down. Each cell undergoes cytokinesis, producing four haploid cells, each with a unique combination of genes and chro- mosomes.
An excellent animation depicting meiosis can be viewed at
http://www.youtube.com/watch?v=D1_-mQS_FZ0&feature=related.
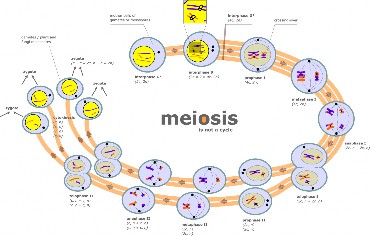
Figure 6.12: Meiosis is a process in which a diploid cell divides itself into four haploid cells. c represents the number of chromosomes, n represents a haploid cell, 2n represents a diploid cell. (16)
Sexual reproduction results in infinite possibilities of genetic variation. This occurs through a number of mechanisms, including crossing-over, the independent assortment of chromosomes during anaphase I, and random fertilization.
Crossing-over occurs during prophase I. Crossing-over is the exchange of genetic material between non-sister chromatids of homologous chromosomes. Recall during prophase I, ho- mologous chromosomes line up in pairs, gene-for-gene down their entire length, forming a configuration with four chromatids, known as a tetrad. At this point, the chromatids are very close to each other and some material from two chromatids switch chromosomes, that is, the material breaks off and reattaches at the same position on the homologous chromosome (Figure 6.13). This exchange of genetic material can happen many times within the same pair of homologous chromosomes, creating unique combinations of genes. This process is also known as recombination.
As mentioned above, in humans there are over 8 million configurations in which the chromo- somes can line up during metaphase I. It is the specific processes of meiosis, resulting in four unique haploid cells, that results in these many combinations. Figure 6.14 compares mitosis and meiosis. This independent assortment, in which the chromosome inherited from either the father or mother can sort into any gamete, produces the potential for tremendous genetic variation. Together with random fertilization, more possibilities for genetic variation exist between any two people than individuals alive today. Sexual reproduction is the random fertilization of a gamete from the female using a gamete from the male. In humans, over 8 million (223) chromosome combinations exist in the production of gametes in both the male
www.ck12.org 308
and female. A sperm cell, with over 8 million chromosome combinations, fertilizes an egg cell, which also has over 8 million chromosome combinations. That is over 64 trillion unique combinations, not counting the unique combinations produced by crossing-over. In other words, each human couple could produce a child with over 64 trillion unique chromosome combinations.
Figure 6.14: Mitosis vs. Meiosis Comparison. Mitosis produces two diploid daugh- ter cells, genetically identical to the parent cell. Meiosis produces four haploid daugh- ter cells, each genetically unique. See How Cells Divide: Mitosis vs. Meiosis (www.pbs.org/wgbh/nova/miracle/divide.html) for an animation comparing the two pro- cesses. (13)
At the end of meiosis, haploid cells are produced. These cells need to further develop into mature gametes capable of fertilization, a process called gametogenesis (Figure 6.15).
www.ck12.org 310
Gametogenesis differs between the sexes. In the male, the production of mature sperm cells, or spermatogenesis, results in four haploid gametes, whereas, in the female, the production of a mature egg cell, oogenesis, results in just one mature gamete.
During spermatogenesis, primary spermatocytes go through the first cell division of meiosis to produce secondary spermatocytes. These are haploid cells. Secondary spermatocytes then quickly complete the meiotic division to become spermatids, which are also haploid cells. The four haploid cells produced from meiosis develop a flagellum tail and compact head piece to become mature sperm cells, capable of swimming and fertilizing an egg. The compact head, which has lost most of its cytoplasm, is key in the formation of a streamlined shape. The middle piece of the sperm, connecting the head to the tail, contains many mitochondria, providing energy to the cell. The sperm cell essentially contributes only DNA to the zygote.
On the other hand, the egg provides the other half of the DNA, but also organelles, building blocks for compounds such as proteins and nucleic acids, and other necessary materials. The egg, being much larger than a sperm cell, contains almost all of the cytoplasm a developing embryo will have during its first few days of life. Therefore, oogenesis is a much more complicated process than spermatogenesis.
Oogenesis begins before birth and is not completed until after fertilization. Oogenesis begins when an oogonia (singular, oogonium), which are the immature eggs that form in the ovaries before birth, with the diploid number of chromosomes undergoes mitosis to form primary oocytes, also with the diploid number. It proceeds as a primary oocyte undergoes the first cell division of meiosis to form secondary oocytes with the haploid number of chromosomes. A secondary oocyte undergoes the second meiotic cell division to form a haploid ovum if it is fertilized by a sperm. The one egg cell that results from meiosis contains most of the cytoplasm, nutrients, and organelles. This unequal distribution of materials produces one
large cell, and one cell with little more than DNA. This other cell, known as a polar body, eventually breaks down. The larger cell undergoes meiosis II, once again producing a large cell and a polar body. The large cell develops into the mature gamete, called an ovum.
Eukaryotes have three different versions of the sexual life cycle: a haploid life cycle, a diploid life cycle, and a life cycle known as the alternation of generations (Figure 6.16). A life cycle is the span in the life of an organism from one generation to the next. All species that reproduce sexually follow a basic pattern, alternating between haploid and diploid chromosome numbers. The sexual life cycle depends on when meiosis occurs and the type of cell that undergoes meiosis.
Figure 6.16: Sexual Life Cycles. (6)
The haploid life cycle is the simplest life cycle. Organisms with this life cycle, such as many protists and some fungi and algae, spend the majority of their life cycle as a haploid cell. In fact, the zygote is the only diploid cell. The zygote immediately undergoes meiosis, producing four haploid cells, which grow into haploid multicellular organisms. These organisms produce gametes by mitosis. The gametes fuse through a process called fusion to produce diploid zygotes which undergo meiosis, continuing the life cycle.
Organisms that have a diploid life cycle spend the majority of their lives as diploid adults. All diploid adults inherit half of their DNA from each parent. When they are ready to reproduce, diploid reproductive cells undergo meiosis and produce haploid gametes. These gametes then fuse through fertilization and produce a diploid zygote, which immediately
www.ck12.org 312
enters G1 of the cell cycle. Next, the zygote’s DNA is replicated. Finally, the processes of mitosis and cytokinesis produce two genetically identical diploid cells. Through repeated rounds of growth and division, this organism becomes a diploid adult and the cycle continues.
Plants, algae, and some protists have a life cycle that alternates between diploid and haploid phases, known as alternation of generations. In plants, the life cycle alternates between the diploid sporophyte and haploid gametophyte. Spore forming cells in the diploid sporophyte undergo meiosis to produce spores, a haploid reproductive cell. Spores can develop into an adult without fusing with another cell. The spores give rise to a multicellular haploid gametophyte, which produce gametes by mitosis. The gametes fuse, producing a diploid zygote, which grow into the diploid sporophyte.
Asexual reproduction produces a clone, an organism that is genetically identical to its parent.
Asexual reproduction includes fission, fragmentation and budding.
Sexual reproduction involves haploid gametes and produces a diploid zygote through fertilization.
Meiosis is a type of cell division that halves the number of chromosomes. There are eight stages of meiosis, divided into meiosis I and meiosis II. DNA is not replicated between meiosis I and meiosis II.
Crossing-over, the independent assortment of chromosomes during anaphase I, and random fertilization result in genetic variation.
Meiosis is a step during spermatogenesis and oogenesis. Spermatogenesis produces four haploid sperm cells, while oogenesis produces one mature ovum.
Eukaryotes have three different versions of the sexual life cycle: a haploid life cycle, a diploid life cycle, and a life cycle known as the alternation of generations. The sexual life cycle depends on when meiosis occurs and the type of cell that undergoes meiosis.
Define crossing-over in meiosis.
Describe how crossing-over, independent assortment, and random fertilization lead to genetic variation.
Compare and contrast mitosis and meiosis.
List the main differences between asexual and sexual reproduction.
How many chromosomes does a diploid human cell have? How many chromosomes does a haploid human cell have?
Name the three different sexual life cycles. What characterizes the differences between these life cycles?
Compare binary fission and asexual reproduction.
http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/M/Meiosis.html
http://www.emc.maricopa.edu/faculty/farabee/BIOBK/BioBookmeiosis.html
alternation of generations A life cycle that alternates between diploid and haploid phases.
asexual reproduction Reproduction without gametes; the simplest and most primitive method of reproduction; produces a clone, an organism that is genetically identical to its parent.
budding Asexual reproduction in which new individuals split off from existing ones; the bud may stay attached or break free from the parent.
crossing-over The exchange of genetic material between non-sister chromatids of homol- ogous chromosomes; also known as recombination.
diploid A cell containing two sets of chromosomes; in human cells, two sets contains 46 chromosomes.
fertilization The joining of gametes during reproduction.
fission Asexual reproduction in which a parent separates into two or more individuals of about equal size.
fragmentation Asexual reproduction in which the body breaks into several fragments, which later develop into complete adults.
gametes An organism’s reproductive cells, such as sperm and egg cells.
gametogenesis The further maturation of the haploid cells produced by meiosis into ma- ture gametes capable of fertilization.
www.ck12.org 314
gametophyte Produces gametes by mitosis; in alternation of generation life cycles.
haploid A cell containing one set of chromosomes; in human gametes, one set is 23 chro- mosomes.
life cycle The span in the life of an organism from one generation to the next.
meiosis A type of cell division that halves the number of chromosomes.
oogenesis The production of a mature egg cell; results in just one mature ovum, or egg cell.
polar body Cell formed during oogenesis; contains little cytoplasm and eventually breaks down; does not form a gamete.
sexual reproduction Reproduction involving the joining of haploid gametes, producing genetically diverse individuals.
spermatogenesis The production of mature sperm cells; results in four haploid gametes.
spore A haploid reproductive cell; found in plants, algae and some protists; can develop into an adult without fusing with another cell.
tetrad A configuration with four chromatids; formed by the pairing of homologous chro- mosomes during prophase I of meiosis.
The next unit, Genetics, discusses the branch of biology that studies heredity. What is heredity?
What role do you think meiosis plays in heredity?
Describe what would happen if gametes were formed by mitosis.
Human Genetics is an ever increasingly important field of medicine. Explain why this field of medicine is so important.
NCBI. http://en.wikipedia.org/wiki/Image:MajorEventsInMitosis.jpg. Public Domain.
CK-12 Foundation. http://en.wikipedia.org/wiki/Image:Chromosomal_Recombination.svg. CC-BY 2.5.
http://en.wikipedia.org/wiki/Image:Sperm-egg.jpg. Public Domain.
http://en.wikipedia.org/wiki/File:Condensed_Eukaryotic_Chromosome.png. GNU-FDL.
CK-12 Foundation. http://en.wikipedia.org/wiki/File:Zygotic_meiosis.png http://en.wikipedia.org/wiki/File:Sporic_meiosis.png Sexual Life Cycles.. GNU-FDL.
Magnification of a budding yeast.. Public Domain.
http://commons.wikimedia.org/wiki/File:Sexual_cycle.svg. GNU-FDL.
Courtesy of: National Human Genome Research Institute. http://www.genome.gov/Pages/Hyperion/DIR/VIP/Glossary/Illustration/ metaphase.cfm?key=metaphase. Public Domain.
CK-12 Foundation. http://en.wikipedia.org/wiki/Image:Gray7.png. Public Domain.
CK-12 Foundation. http://en.wikipedia.org/wiki/Image: Normal_cancer_cell_division_from_NIH.png. Public Domain.
USNLM. http://ghr.nlm.nih.gov/handbook/illustrations/mitosismeiosis. Public Domain.
http://en.wikipedia.org/wiki/Image:Binary_fission.png. CC-BY-SA 3.0.
http://commons.wikimedia.org/wiki/File: Cytokinesis-electron-micrograph.jpg. Public Domain.
Marek Kultys. http://en.wikipedia.org/wiki/File:Meiosis_diagram.jpg. CC-BY-SA.
www.ck12.org 316
Identify how Mendel’s study of science and math was important to his success in research.
Distinguish between characteristics and traits.
Explain how Mendel was able to control pollination of the pea plants.
Identify the terms used to describe the three generations in Mendel’s studies.
State one reason for carrying out a monohybrid cross.
Identify the traits that appeared in Mendel’s F2 generation.
Identify the actions of dominant alleles and recessive alleles for a trait.
Outline the Law of Segregation.
Outline the Law of Independent Assortment.
Explain Mendel’s results in relation to genes and chromosomes.
Distinguish between genotype and phenotype.
For thousands of years, humans have understood that characteristics such as eye color or flower color are passed from one generation to the next. The passing of characteristics from parent to offspring is called heredity. Humans have long been interested in understanding heredity. Many hereditary mechanisms were developed by scholars but were not properly tested or quantified. The scientific study of genetics did not begin until the late 19th century. In experiments with garden peas, Austrian monk Gregor Mendel described the patterns of inheritance.
Gregor Johann Mendel was an Augustinian monk, a teacher, and a scientist (Figure 7.1). He is often called the ”father of modern genetics” for his study of the inheritance of traits in pea plants. Mendel showed that the inheritance of traits follows particular laws, which were later named after him. The significance of Mendel’s work was not recognized until the turn of the 20th century. The rediscovery of his work led the foundation for the era of modern genetics, the branch of biology that focuses on heredity in organisms.

Figure 7.1: Gregor Johann Mendel “The Father of Modern Genetics.” 1822-1884. (3)
Johann Mendel was born in 1822 and grew up on his parents’ farm in an area of Austria that is now in the Czech Republic. He overcame financial hardship and ill health to excel in school. In 1843 he entered the Augustinian Abbey in Brünn (now Brno, Czech Republic.) Upon entering monastic life, he took the name Gregor. While at the monastery, Mendel also attended lectures on the growing of fruit and agriculture at the Brünn Philosophical Institute. In 1849 he accepted a teaching job, but a year later he failed the state teaching examination. One of his examiners recommended that he be sent to university for further studies. In 1851 he was sent to the University of Vienna to study natural science and mathematics. Mendel’s time at Vienna was very important in his development as a scientist. His professors encouraged him to learn science through experimentation and to use mathematics to help explain observations of natural events. He returned to Brünn in 1854 as a natural history and physics teacher.
www.ck12.org 318
In 1853 and 1854, Mendal published two papers on crop damage by insects. However, he is best known for his later studies of the pea plant Pisum sativum. Mendel was inspired by both his professors at university and his colleagues at the monastery to study variation in plants. He had carried out artificial fertilization on plants many times in order to grow a plant with a new color or seed shape. Artificial fertilization is the process of transferring pollen from the male part of the flower to the female part of another flower. Artificial fertilization is done in order to have seeds that will grow into plants that have a desired trait, such as yellow flowers.
During Mendel’s time, the popular blending inheritance hypothesis stated that offspring were a ”mix” of their parents. For example, if a pea plant had one short parent and one tall parent, that pea plant would be of medium height. It was believed that the offspring would then pass on heritable units, or factors, for medium sized offspring. (Today we know these heritable units are genes; however, Mendel did not know of the concept of a gene.) Mendel noted that plants in the monastery gardens sometimes gave rise to plants that were not exactly like the parent plants, nor were they a “mix” of the parents. He also noted that certain traits reappeared after “disappearing” in an earlier generation. Mendel was interested in finding out if there was a predictable pattern to the inheritance of traits. Between 1856 and 1863 he grew and tested about 29,000 pea plants in the monastery garden.
Mendel may have chosen to study peas because they are fast-growing plants that are available in different varieties. For example, one variety of pea plant has purple flowers, as shown in Figure 7.2, while another variety has white flowers.
Mendel chose to study seven characteristics of pea plants. A characteristic is a herita- ble feature, such as flower color. Each characteristic Mendel chose to study occurred in two contrasting traits. A trait is a heritable variant of a characteristic, such as purple or white flower color. Table 7.1 lists the seven characteristics Mendel studied, and their two contrasting traits.
Flower Color | Flower Position | Stem Length | Pod Shape | Pod Color | Seed | Seed Color |
violet-red | axial | tall | inflated | green | round | green |
white | terminal | short | constricted | yellow | wrinkled | yellow |
Table 7.1: The Seven Characteristics Mendel Studied and Their Contrasting Traits
on Stem
Shape
(purple)

Figure 7.2: Pisum sativum, the pea plant species that Mendel studied. (2)
In order to study these characteristics, Mendel needed to control the pollination of the pea plants. Pollination occurs when the pollen from the male reproductive part of a flower, called the anthers, is transferred to the female reproductive part of a flower, called the stigma. Pea plants are self-pollinating, which means the pollen from a flower on a single plant transfers to the stigma of the same flower or another flower on the same plant. In order to avoid self-pollination, Mendel removed the anthers from the flowers on a plant. He then carefully transferred pollen from the anthers of another plant and dusted the pollen onto the stamen of the flowers that lacked anthers. This process caused cross-pollination. Cross-pollination occurs when pollen from one flower pollinates a flower on a different plant. In this way, Mendel controlled the characteristics that were passed onto the offspring. Figure 7.3 shows the location of the male and female parts of P. sativum.
Mendel began his studies by growing plants that were true-breeding for a particular trait. A true-breeding plant will always produce offspring with that trait when they self-pollinate. For example, a true-breeding plant with yellow seeds will always have offspring that have yellow seeds. In his first experiment, Mendel cross-pollinated two true-breeding plants of contrasting traits, such as purple and white flowered plants. The true-breeding parent plants are referred to as the P generation (parental generation). The hybrid offspring of the P
www.ck12.org 320

generation are called the F1 generation (filial generation). The hybrid offspring of the F1 generation are called the F2 generation (filial generation).
Mendel first worked with plants that differed in a single characteristic, such as flower color. A hybridization is a cross between two individuals that have different traits. A hybridization in which only one characteristic is examined is called a monohybrid cross. The offspring of such a cross are called monohybrids. Mendel noted that hybridizing true-breeding (P- generation) plants gave rise to an F1 generation that showed only one trait of a characteristic. For example, a true-breeding purple-flowering plant crossed with a true-breeding white- flowering plant always gave rise to purple-flowered hybrid plants. There were no white- flowered hybrids! Mendel wanted to know what happened to the white-flowered plants’ “heritable factors.” If indeed the white-flower “heritable factor” had disappeared, all future offspring of the hybrids would be purple-flowered. To test this idea, Mendel let the F1 generation plants self-pollinate and then planted the resulting seeds.
The F2 generation plants that grew included white-flowered plants! Mendel noted the ratio of white flowered plants to purple-flowered plants was about 3:1. That is, for every three purple-flowered plants, there was one white flowered plant. Figure 7.4 shows Mendel’s results for the characteristic of flower color.
Mendel carried out identical studies over three generations, (P, F1, and F2), for the other six characteristics and found in each case that one trait “disappeared” in the F1 generation, only to reappear in the F2 generation. Mendel studied a large number of plants, as shown in Table 7.2, so he was confident that the ratios of different traits in the F2 generation were representative.
Characteristic | Dominant Trait | Recessive Trait | F2 Generation Ratio Dominant:Recessive | |
Flower color | Purple | White | 705:224 | 3.15:1 |
Flower position | Axial | Terminal | 651:207 | 3.14:1 |
Stem length | Tall | Short | 787:277 | 2.84:1 |
Pod shape | Inflated | Constricted | 882:299 | 2.95:1 |
Pod color | Green | Yellow | 428:152 | 2.82:1 |
322 | ||||
Table 7.2: Results of F1 Generation Crosses for Seven Characteristics in
on stem
Table 7.2: (continued)
![]()
Characteristic Dominant Trait Recessive Trait F2 Generation Ratio
Dominant:Recessive
![]()
Seed shape Round Wrinkled or an- gular
5474:1850 2.96:1
Seed color Yellow Green 6022:2001 3.01:1
![]()
Based on his observations, Mendel developed four hypotheses. These hypotheses are known as Mendel’s theory of heredity. The hypotheses explain a simple form of inheritance in which two alleles of a gene are inherited to result in one of several traits in offspring. In modern terms, these hypotheses are:
There are different versions of genes. These different versions account for vari- ations in characteristics. Different versions of a gene are called alleles. For example, there is a “yellow-pod” allele and a “green pod” allele. The blending inheritance hy- pothesis was discredited by Mendel’s allele hypothesis.
When two different alleles are inherited together, one may be expressed, while the effect of the other may be “silenced.” In the case of pod color, the allele for green pods is always expressed and is dominant. The allele for yellow pods, which is not expressed, is recessive. For instance, if a plant inherits a “yellow-pod” gene and a “green pod” gene, it will have only green pods.
For each characteristic, an organism inherits two alleles, one from each parent. Mendel noted that offspring could inherit their traits from either parent. In the case of the expressed trait, it did not matter whether it was the male gamete or female gamete that supplied the gene.
When gametes are formed, the two alleles of each gene are separated (Figure 7.5). During meiosis, each male or female gamete receives one allele for a trait. When the male and female gametes are fused at fertilization, the resulting zygote contains two alleles of each gene.
The Law of Segregation states that a pair of alleles is separated, or segregated, during the formation of gametes. During meiosis, homologous chromosomes are randomly separated. Each resulting gamete has an equal probability or chance of receiving either of the two alleles.
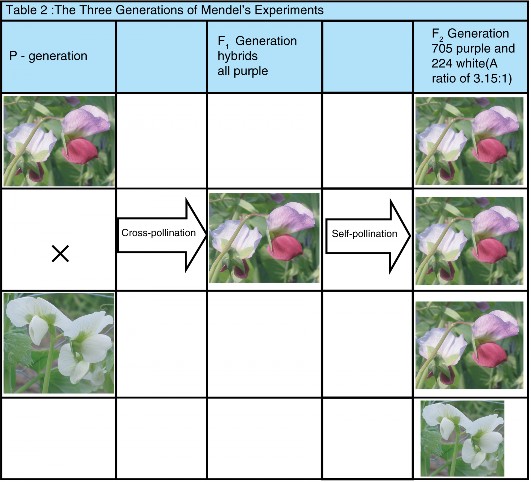
www.ck12.org 324
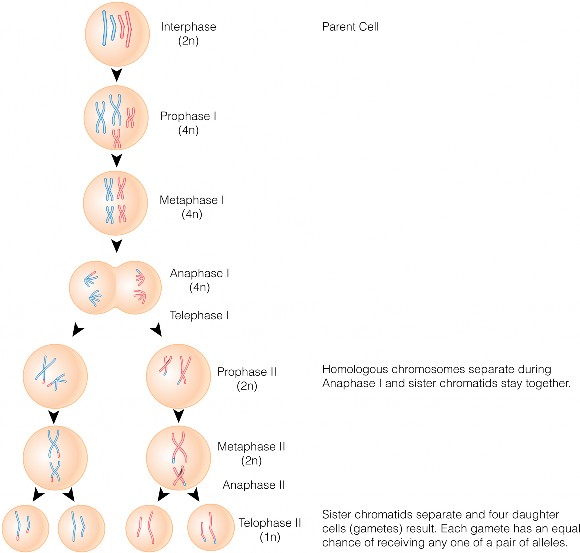
Mendel also crossed pea plants that differed in two characteristics, such as seed color and shape. A dihybrid cross is a cross in which the inheritance of two characteristics are tracked at the same time. The offspring of such a cross are called dihybrids. Mendel wanted to see if the inheritance of characteristics were dependent. He concluded that characteristics were inherited independently of each other.
The Law of Independent Assortment, also known as or Mendel’s Second Law, states that the inheritance of one trait will not affect the inheritance of another. Mendel concluded that different traits are inherited independently of each other, so that there is no relationship, for example, between seed color and seed shape. In modern terms, alleles of each gene separate independently during gamete formation.
We now know that the only alleles that are inherited independently are ones that are located far apart on a chromosome or that are on different chromosomes. There are many genes that are close together on a chromosome, and are packaged into the gametes together. Genes that are inherited in this way are called linked genes. Linked genes tend to be inherited together because they are located on the same chromosome. Genetic linkage was first discovered by the British geneticists William Bateson and Reginald Punnett shortly after Mendel’s laws were rediscovered.
Mendel was perhaps lucky in that the characteristics he chose to study in the pea plants had a relatively simple pattern of inheritance. These characteristics were determined by one gene for which there were exactly two alleles. One of these alleles was dominant and the other recessive. Had any of these characteristics been determined by more than one gene, he may not have been able to develop such amazing insight into inheritance. In many instances, the relationship between genes and inheritance is more complex than that which Mendel found. Nevertheless, geneticists have since found that Mendel’s findings can be applied to many organisms. For example, there are clear patterns of Mendelian inheritance in humans. Albinism (Figure 7.6), is a genetic disorder that is inherited as a simple Mendelian trait.
www.ck12.org 326

Mendel used letters to represent dominant and recessive factors. Likewise, geneticists now use letters to represent alleles. Capital letters refer to dominant alleles, and lowercase letters refer to recessive alleles. For example, the dominant allele for the trait of green pod color is indicated by G. The recessive trait of yellow pod color is indicated by g. A true-breeding plant for green pod color would have identical alleles GG in all its somatic cells. Likewise, a true-breeding plant for yellow pod color would have identical alleles gg in all of its somatic cells. During gamete formation, each gamete receives one copy of an allele. When fertilization occurs between these plants, the offspring receives two copies of the allele, one from each parent. In this case, all of the offspring would have two different alleles, Gg, one from each of its parents.
An organism that has an identical pair of alleles for a trait is called homozygous. The true- breeding parents GG and gg are homozygous for the pod color gene. Organisms that have two different alleles for a gene are called heterozygous. The offspring of the cross between the GG and gg plants are all heterozygous for the pod color gene. Due to dominance and recessiveness of alleles, an organism’s traits do not always reveal its genetics. Therefore, geneticists distinguish between an organism’s genetic makeup, called its genotype, and its physical traits, called its phenotype. For example, the GG parent and the Gg offspring have the same phenotype (green pods) but different genotypes.
Genetics is the branch of biology that focuses on heredity in organisms.
Modern genetics is based on Mendel’s explanation of how traits are passed from gen- eration to generation.
Mendel’s use of mathematics in his pea plant studies was important to the confidence he had in his results.
Mendel carried out his first experiments with true-breeding plants and continued them over a span of three generations.
For each of the seven characteristics Mendel studied, he observed a similar ratio in the inheritance of dominant to recessive traits (3:1) in the F2 generation.
Mendel developed a theory that explained simple patterns of inheritance in which two alleles are inherited to result in one of several traits in offspring.
The law of segregation states that a pair of alleles is segregated during the formation of gametes and that each gamete has an equal chance of getting either one of the allele.
The law of independent assortment states that the inheritance of one trait will not affect the inheritance of another. That is, genes are inherited independently of each other.
Linked genes are genes that are close together on the same chromosome. Linked genes are inherited together.
Mendelian inheritance patterns can be seen in humans. Albinism is a genetic disorder that is inherited as a simple Mendelian trait.
Genotype determines phenotype. A homozygous dominant or a heterozygous genotype will always show a dominant phenotype. A homozygous recessive genotype can only show a recessive phenotype.
Why was Mendel’s understanding of mathematics and science important for his re- search?
What did Gregor Mendel contribute to the science of genetics?
What is a true-breeding plant?
How was Mendel able to control the pollination of his pea plants?
How does cross-pollination differ from self-pollination?
How did the appearance of Mendel’s F1 generation differ from the appearance of the P generation?
Identify the relationship between genes and alleles.
Summarize the law of segregation.
Summarize the law of independent assortment.
Relate the term homozygous to heterozygous by using an example from Mendel’s ex- periments.
Relate the term genotype to phenotype by using an example from Mendel’s experi- www.ck12.org 328
ments.
Why can’t you always identify the genotype of an organism from its phenotype?
http://www.macalester.edu/psychology/whathap/UBNRP/visionwebsite04/twotypes. html
http://evolution.berkeley.edu/evosite/history/discretegenes.shtml
allele Different versions of a gene.
anther The male reproductive part of a flower.
artificial fertilization The process of transferring pollen from the male part of the flower to the female part of another flower; done in order to have seeds that will grow into plants that have a desired trait.
blending inheritance hypothesis Hypothesis that stated that offspring were a ”mix” of their parents.
characteristic A heritable feature, such as flower color.
cross-pollination Fertilization in which pollen from one flower pollinates a flower on a different plant.
dihybrid cross A cross in which the inheritance of two characteristics are tracked at the same time.
dominant The allele that is expressed when two separate alleles are inherited.
F1 generation The hybrid offspring of the P (parental) generation; first filial generation.
genetics The branch of biology that focuses on heredity in organisms.
genotype An organism’s genetic makeup.
heredity The passing of characteristics from parent to offspring. heterozygous Organisms that have two different alleles for a gene. homozygous An organism that has an identical pair of alleles for a trait. hybridization A cross between two individuals that have different traits.
Law of Independent Assortment States that the inheritance of one trait will not affect the inheritance of another.
Law of Segregation States that a pair of alleles is separated, or segregated, during the formation of gametes.
linked genes Genes that are close together on a chromosome, and are packaged into the gametes together.
monohybrid cross A hybridization in which only one characteristic is examined.
phenotype An organism’s physical traits.
recessive The allele that is expressed only in the absence of a dominant allele.
self-pollinating Fertilization in which the pollen from a flower on a single plant transfers to the stigma of the same flower or another flower on the same plant.
stigma The female reproductive part of a flower.
trait A heritable variant of a characteristic, such as purple or white flower color.
true-breeding A plant that will always produce offspring with the parental trait when it self-pollinates.
www.ck12.org 330
Next we will examine Mendelian Inheritance in further detail.
Do you think all inheritance is as straightforward as the inheritance in pea plants?
Is there a relationship between inheritance and probability? What might that rela- tionship be?
Identify how probability is used to predict outcomes of genetic crosses.
Outline how a Punnett Square helps predict outcomes of genetic crosses.
Identify how probability can help determine the alleles in a gamete.
Identify how a testcross is used to determine the genotype of an organism.
Describe how monohybrid and dihybrid crosses differ.
Identify the ratio of phenotypes that appeared in Mendel’s dihybrid crosses.
Examine how a pedigree is used in the study of human inheritance.
Describe how codominance does not follow Mendelian Inheritance.
Describe how incomplete dominance does not follow Mendelian Inheritance.
Identify examples of polygenic traits in humans.
Outline how heredity and environment can interact to affect phenotype.
A Mendelian trait is a trait that is controlled by a single gene that has two alleles. One of these alleles is dominant and the other is recessive. Several inheritable conditions in humans are passed to offspring in a simple Mendelian fashion. Medical professionals use Mendel’s laws to predict and understand the inheritance of certain traits in their patients. Also, farmers, animal breeders, and horticulturists who breed organisms can predict outcomes of crosses by understanding Mendelian inheritance.
The rules of probability that apply to tossing a coin or throwing a dice also apply to the laws of segregation and independent assortment. Probability is the likelihood that a certain event will occur. It is expressed by comparing the number of events that occur to the total number of possible events. The equation is written as:
Probability = (number of times an event is expected to occur/total number of times an event could happen)
For example, in Mendel’s F2 hybrid generation, the dominant trait of purple flower color ap- peared 705 times, and the recessive trait appeared 224 times. The dominant allele appeared 705 times out of a possible 929 times (705+224=929).
Probability = (705/929) (705/929)= 0.76
The probability the recessive trait will appear in the F2 hybrid generation is calculated in the same way.
Probability = (224/929) (224/929) = 0.24
The probability of the recessive trait appearing in the F2 generation is 24% or about ¼.
Results predicted by probability are most accurate when many trials are done. The best way to illustrate this idea is to toss a coin. Because a coin has two sides, every time you toss it the chance of tossing heads or tossing tails is 50%. The outcome of each separate toss is unaffected by any previous or future result. For example, imagine you tossed seven heads in a row. You would think that the next toss is more likely to be a tail, but the possibility of tossing another head is still 50%. If you tossed the coin a total of ten times, a total of seven heads and three tails, you would calculate the probability of tossing heads is 70%. The fact that you carried out only a small number of trials has affected your results. If Mendel had grown only 10 plants, he would have gotten different probabilities for the appearance of dominant and recessive traits. However, Mendel carried out many thousands of trials. He was therefore sure that his results were due to probability, and not to chance.
Each coin toss is a separate event. Likewise, gamete formation is a separate event. The probability that a Pp heterozygote produces gametes with a P allele or a p allele is 50% for each gamete cell. In a fertilization involving two such plants (as in the F1 generation self-pollination experiment), each pollen cell and each egg cell have a 50% chance of having the P or p allele.
www.ck12.org 332
Mendel developed the law of segregation by following only a single characteristic, such as pod color, in his pea plants. Biologists use a diagram called a Punnett Square, to help predict the probable inheritance of alleles in different crosses. In a monohybrid cross, such as the one in Figure 7.7, the Punnett square shows every possible combination when combining one maternal (mother) allele with one paternal (father) allele. In this example, both organisms are heterozygous for flower color Pp (purple). Both plants produce gametes that contain both the P and p alleles. The probability of any single offspring showing the dominant trait is 3:1, or 75%.

In the monohybrid cross shown in Figure 7.7, two heterozygous plants are crossed. Both plants produce gametes, all of which contain either a P or p allele for flower color. The likelihood that any particular gamete contains the allele for a white flower can be calculated. Because a gamete can only carry one out of two alleles, there are only two possible outcomes
for a gamete. The probability that a gamete will carry the allele for white flower color is ½, 0.5, or 50%. The probability that a gamete will carry the allele for purple flower color is also
½.
We can calculate the probability of any one of the offspring being heterozygous (Pp) or homozygous (PP or pp) for flower color. The probability of a plant inheriting the P or p allele from a heterozygous parent is ½. Multiply the probabilities of inheriting both alleles to find the probability that any one plant will be a pp homozygote.
½ × ½ = ¼ or 0.25
Only 25 %, or one outcome out of four, will result in a plant homozygous for white flower color (pp). The possibility that any one plant will be a PP homozygote is also 1/4. The heterozygous allele combination can happen twice (Pp or pP), so the two probabilities are added together ¼ + ¼ = 2/4, or ½. The probability that an offspring plant will be Pp heterozygous is ½.
Suppose you have a purple and white flower and, as discussed above, purple color is dominant to white. The white flower must be homozygous for the recessive allele, but the genotype of the purple flower is unknown. It could be either PP or Pp. A testcross will determine the organism’s genotype. In a testcross, the individual with the unknown genotype is crossed with a homozygous recessive individual (Figure 7.8). The unknown genotype can be determined by observing the phenotypes of the resulting offspring.
Dihybrid crosses are more complicated than monohybrid crosses because more combinations of alleles are possible. For example, tracking the inheritance of seed color and pod color in a Punnett square requires that we track four alleles. G is the dominant allele for green pod color and g is the recessive allele for yellow pods. Y is the dominant allele for yellow seed color and y is the recessive allele for green seed color.
Two plants are crossed, one is true-breeding for green pods and yellow seeds (GGYY), the other is true-breeding for yellow pods and green seeds (ggyy). All of the F1 generation will be heterozygous for both traits (GgYy). Figure 7.9, shows the dihybrid cross of the dihybrid P generation and the F1 generation.
www.ck12.org 334
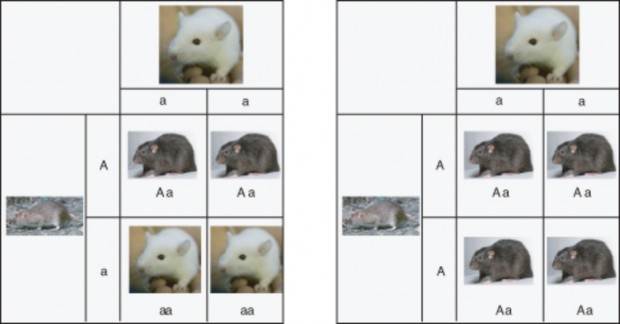
Figure 7.8: A testcross helps reveal the genotype of an organism when that organism shows the dominant trait, such as agouti coat color in rats. Such an organism could be homozygous dominant or heterozygous. Agouti is the common color of the Norway rat, Rattus norvegicus. (12)
In a dihybrid cross, four alleles can be inherited from any one parent at one time. When two heterozygous individuals are crossed, there are a total of 16 possible combinations of the four alleles. The phenotypes of the offspring with two independent traits show a 9:3:3:1 ratio. In a cross involving pea plants heterozygous for round, yellow seeds (GgYy), 9/16 plants have round, yellow seeds, 3/16 have round, green seeds, 3/16 have wrinkled, yellow seeds, and 1/16 has wrinkled, green seeds.
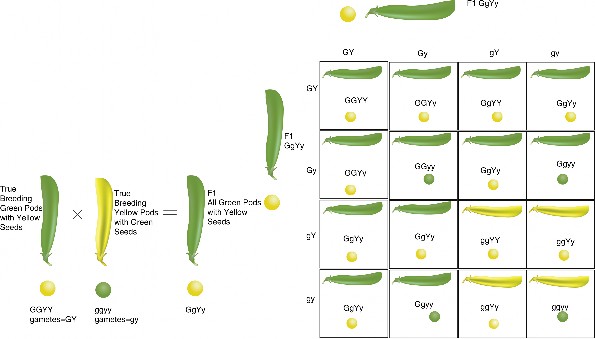
A pedigree is a chart which shows the inheritance of a trait over several generations. A pedigree is commonly created for families, and it outlines the inheritance patterns of genetic disorders. Figure 7.10 shows a pedigree depicting recessive inheritance of a disorder through three generations. Scientists can tell the genetics of inheritance from studying a pedigree, such as whether the trait is sex-linked (on the X or Y chromosome) or autosomal (on a chromosome that does not determine sex), whether the trait is inherited in a dominant
www.ck12.org 336
or recessive fashion, and possibly whether individuals with the trait are heterozygous or homozygous.
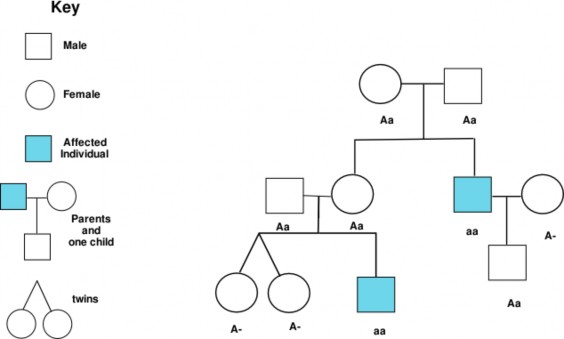
Is the trait sex-linked or autosomal? A sex chromosome is a chromosome that determines the sex of an organism. Humans have two sex chromosomes, X and Y. Females have two X chromosomes (XX), and males have one X and one Y (XY). An autosome is any chromosome other than a sex chromosome. If a trait is autosomal it will affect males and females equally.
A sex-linked trait is a trait whose allele is found on a sex chromosome. The human X chromosome is significantly larger than the Y chromosome; there are many more genes located on the X chromosome than there are on the Y chromosome. As a result there are many more X-linked traits than there are Y-linked traits. Most sex-linked traits are recessive. Because males carry only one X chromosome, if they inherit a recessive sex-linked gene they will show a sex-linked condition.
Because of the recessive nature of most sex-linked traits, a female who shows a sex-linked condition would have to have two copies of the sex-linked allele, one on each of her X
chromosomes. Figure 7.11 shows how red-green colorblindness, a sex-linked disorder, is passed from parent to offspring.
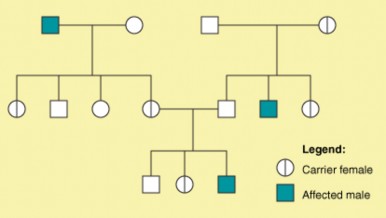
Is the Trait Dominant or Recessive? If the trait is autosomal dominant, every person with the trait will have a parent with the trait. If the trait is recessive, a person with the trait may have one, both or neither parent with the trait. An example of an autosomal dominant disorder in humans is Huntington’s disease (HD). Huntington’s disease is a degenerative disease of the nervous system. It has no obvious effect on phenotype until the person is aged 35 to 45 years old. The disease is non-curable and, eventually, fatal. Every child born to a person who develops HD has a 50% chance of inheriting the defective allele from the parent.
Are the Individuals with the Trait Heterozygous or Homozygous? If a person is homozygous or heterozygous for the dominant allele of a trait, they will have that trait. If the person is heterozygous for a recessive allele of the trait, they will not show the trait. A person who is heterozygous for a recessive allele of a trait is called a carrier. Only people who are homozygous for a recessive allele of a trait will have the trait.
The relationship between genotype and phenotype is rarely as simple as the examples Mendel studied. Each characteristic he studied had two alleles, one of which was completely domi- nant and the other completely recessive. Geneticists now know that alleles can be codomi- nant, or incompletely dominant.
www.ck12.org 338
Codominance occurs when both traits appear in a heterozygous offspring. Neither allele is completely dominant nor completely recessive. For example, roan shorthorn cattle have codominant genes for hair color. The coat has both red and white hairs. The letter R indicates red hair color, and R’ white hair color. In cases of codominance, the genotype of the organism can be determined from its phenotype. The heifer in Figure 7.12 is RR’ heterozygous for coat color.

Incomplete dominance occurs when the phenotype of the offspring is somewhere in between the phenotypes of both parents; a completely dominant allele does not occur. For example, when red snapdragons (CRCR)</math> are crossed with white snapdrag- ons (CWCW), the F1 hybrids are all pink hetrozygotes for flower color (CRCW). The pink color is an intermediate between the two parent colors. When two F1 (CRCW) hybrids are crossed they will produce red, pink, and white flowers. The genotype of an organism with incomplete dominance can be determined from its phenotype (Figure 7.13).
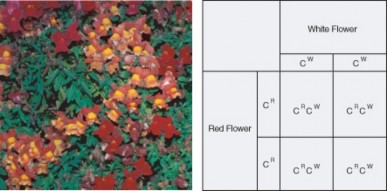
Traits that are affected by more than one gene are called polygenic traits. The genes that affect a polygenic trait may be closely linked on a chromosome, unlinked on a chromosome, or on different chromosomes. Polygenic traits are often difficult for geneticists to track because the polygenic trait may have many alleles. Also, independent assortment ensures the genes combine differently in gametes. Therefore, many different intermediate phenotypes exist in offspring. Eye color (Figure 7.14), and skin color are examples of polygenic traits in humans.
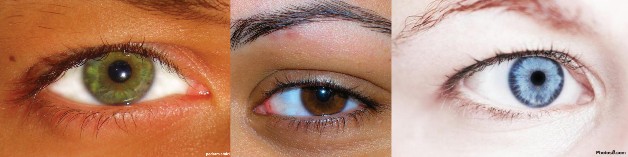
When three or more alleles determine a trait, the trait is said to have multiple alleles. The human ABO blood group is controlled by a single gene with three alleles: i, IA, IB, and the recessive i allele. The gene encodes an enzyme that affects carbohydrates that are found on the surface of the red blood cell. A and B refer to two carbohydrates found on the surface of red blood cells. There is not an O carbohydrate. Type O red blood cells do not have either
www.ck12.org 340
type A or B carbohydrates on their surface.
The alleles IA and IB are dominant over i. A person who is homozygous recessive ii has type O blood. Homozygous dominant IAIA or heterozygous dominant IAi have type A blood, and homozygous dominant IBIB or heterozygous dominant IBi have type B blood. IAIB people have type AB blood, because the A and B alleles are codominant. Type A and type B parents can have a type AB child. Type A and a type B parent can also have a child with Type O blood, if they are both heterozygous (IBi,IAi). The table (7.3) shows how the different combinations of the blood group alleles can produce the four blood groups, A, AB, B, and O.
Table 7.3: Bloodtype as Determined by Multiple Alleles
![]()
IA IB i
![]()
IA IAIA
TYPE A
IB IA IB
TYPE AB
i i IA
TYPE A
IAIB TYPE AB
IB IB TYPE B
i IB TYPE B
IAi TYPE A
IB i TYPE B
ii
TYPE O
![]()
Genes play an important part in influencing phenotype, but genes are not the only influ- ence. Environmental conditions, such as temperature and availability of nutrients can affect phenotypes. For example, temperature affects coat color in Siamese cats.
The pointed pattern is a form of partial albinism, which results from a mutation in an enzyme that is involved in melanin production. The mutated enzyme is heat-sensitive; it fails to work at normal body temperatures. However, it is active in cooler areas of the skin. This results in dark coloration in the coolest parts of the cat’s body, such as the lower limbs and the face, as shown in Figure 7.15. The cat’s face is cooled by the passage of air through the nose. Generally adult Siamese cats living in warm climates have lighter coats than those in cooler climates.
Height in humans is influenced by many genes, but is also influenced by nutrition. A person who eats a diet poor in nutrients will not grow as tall as they would have had they eaten a more nutritious diet. Scientists often study the effects of environment on phenotype by studying identical twins. Identical twins have the same genes, so phenotypic differences between twins often have an environmental cause.

www.ck12.org 342
Probability is the likelihood that a certain event will occur. It is expressed by compar- ing the number of events that actually occur to the total number of possible events. Probability can be expressed as a fraction, decimal, or ratio.
A Punnett square shows all the possible genotypes that can result from a given cross.
A testcross examines the genotype of an organism that shows the dominant phenotype for a given trait. The heterozygous organism is crossed with an organism that is homozygous recessive for the same trait.
A dihybrid cross-examines the inheritance of two traits at the same time.
A pedigree can help geneticists discover if a trait is sex-linked, if it is dominant or reces- sive, and if the person (or people) who have the trait are homozygous or heterozygous for that trait.
The Mendelian pattern of inheritance and expression does not apply to all traits. Codominant traits, incompletely dominant traits, and polygenic traits do not follow simple Mendelian patterns of inheritance. Their inheritance patterns are more complex.
An organism’s phenotype can be influenced by environmental conditions.
What does the probability equation help to determine?
How can probability be expressed?
Outline how Punnett squares are useful.
Identify the purpose of a testcross.
How do the Punnett squares for a monohybrid cross and a dihybrid cross differ?
Mendel carried out a dihybrid cross to examine the inheritance of the characteristics for seed color and seed shape. The dominant allele for yellow seed color is Y, and the recessive allele for green color is y. The dominant allele for round seeds is R, and the recessive allele for a wrinkled shape is r. The two plants that were crossed were F1 dihybrids RrYy. Identify the ratios of traits that Mendel observed in the F2 generation, and explain in terms of genotype what each number means. Create a Punnett square to help you answer the question.
Draw a pedigree that illustrates the passing of the dominant cleft chin trait through three generations. A person who has two recessive alleles does not have a cleft chin. Let us say that C is the dominant allele, c is the recessive allele.
A classmate tells you that a person can have type AO blood. Do you agree? Explain.
Mendelian inheritance does not apply to the inheritance of alleles that result in incom- plete dominance and codominance. Explain why this is so.
Outline the relationship between environment and phenotype.
http://www.nbii.gov/portal/server.pt?open=512&amp;objID=405&amp; PageID=581&amp;mode=2&amp;in_hi_userid=2&amp;cached=true
http://www.macalester.edu/psychology/whathap/UBNRP/visionwebsite04/twotypes. html
http://www.hhmi.org/biointeractive/vlabs/cardiology/content/dtg/pedigree/ pedigree.html
http://www.ndsu.nodak.edu/instruct/mcclean/plsc431/mendel/mendel9.htm
http://www.emc.maricopa.edu/faculty/farabee/BIOBK/BioBookgeninteract.html
autosome Any chromosome other than a sex chromosome.
carrier A person who is heterozygous for a recessive allele of a trait.
codominance Occurs when both traits appear in a heterozygous individual.
incomplete dominance Occurs when the phenotype of the offspring is somewhere in be- tween the phenotypes of both parents; a completely dominant allele does not occur.
Mendelian trait A trait that is controlled by a single gene that has two alleles.
multiple alleles When three or more alleles determine a trait, such as with the human ABO blood group.
probability The likelihood that a certain event will occur.
pedigree A chart which shows the inheritance of a trait over several generations.
polygenic traits Traits that are affected by more than one gene.
Punnett square A diagram that helps predict the probable inheritance of alleles in dif- ferent crosses.
www.ck12.org 344
sex chromosome A chromosome that determines the sex of an organism. sex-linked trait A trait whose allele is found on a sex chromosome. testcross A cross used to determine an unknown genotype.
The next chapter is Molecular Genetics.
What do you think Molecular Genetics refers to?
How can DNA contain all the genetic information?
If DNA is in the nucleus, and proteins are made on ribosomes in the cytoplasm, how do you think this happens?
CK-12 Foundation. http://commons.wikimedia.org/wiki/Image: Blauwschokker_Kapucijner_rijserwt_bloem_Pisum_sativum.jpg. GNU-FDL.
Bmdavll. http://en.wikipedia.org/wiki/Image:Snow_pea_flowers.jpg. GNU-FDL.
http://commons.wikimedia.org/wiki/Image:Mendel.png. Public Domain.
Rozzychan. http://en.wikipedia.org/wiki/Image:PedigreechartB.png. CC-BY-SA 2.5.
CK-12 Foundation,Forest & Kim Starr.
http://commons.wikimedia.org/wiki/File:
Starr_980630-1518_Antirrhinum_majus.jpg. CC-BY 3.0.
CK-12 Foundation. http://commons.wikimedia.org/wiki/Image: Blauwschokker_Kapucijner_rijserwt_bloem_Pisum_sativum.jpg. GNU-FDL.
Robert Scarth. http://www.flickr.com/photos/robert_scarth/63966084/. CC-BY-SA 2.0.
http://commons.wikimedia.org/wiki/Image:Illustration_Pisum_sativum0.jpg. Public Domain.
http://commons.wikimedia.org/wiki/File:Niobe050905-Siamese_Cat.jpeg. GNU-FDL.
Bertas la Blanquita. http://commons.wikimedia.org/wiki/File:Albino_girl_honduras.jpg. CC-BY-SA.
CK-12 Foundation. http://www.flickr.com/photos/forbiddendoughnut/462468304/in/datetaken/ http://www.flickr.com/photos/alexk100/444028894/in/datetaken/.
CC-BY-SA 2.0.
http://www.flickr.com/photos/marypaulose/292958125/ http://www.flickr.com/photos/publicdomainphotos/3478083781/. CC-BY-SA, CC-BY.
www.ck12.org 346
Discuss how the work of Griffith, Avery, Hershey, and Chase demonstrated that DNA is the genetic material.
Define transformation and explain that transformation is the change in genotype and phenotype due to the assimilation of the external DNA by a cell.
Discuss the findings of Chargaff. Describe the importance of the finding that in DNA, the amount of adenine and thymine were about the same and that the amount of guanine and cytosine were about the same. This finding lead to the base pairing rules.
Explain Watson and Crick’s double helix model of DNA.
Describe how DNA is replicated.
Explain the importance of the fact that during DNA replication, each strand serves as a template to make a complementary DNA strand.
Describe the structure and function of RNA.
Discuss the role of the three types of RNA: mRNA, rRNA, and tRNA.
What tells the first cell of an organism what to do? How does that first cell know to become two cells, then four cells, and so on? Does this cell have instructions? What are those instructions and what do they really do? What happens when those instructions don’t work properly? Are the “instructions” the genetic material? Though today it seems completely obvious that Deoxyribonucleic acid, or DNA, is the genetic material, this was not always known.
Practically everything a cell does, be it a liver cell, a skin cell, or a bone cell, it does because of proteins. It is your proteins that make a bone cell act like a bone cell, a liver cell act like a liver cell, or a skin cell act like a skin cell. In other words, it is the proteins that give an organism its traits. We know that it is your proteins that that make you tall or short, have light or dark skin, or have brown or blue eyes. But what tells those proteins how to act? It is the structure of the protein that determines what it does. And it is the order and type of amino acids that determine the structure of the protein. And that order and type of amino acids that make up the protein are determined by your DNA sequence.
The relatively large chromosomes that never leave the nucleus are made of DNA. And, as proteins are made on the ribosomes in the cytoplasm, how does the information encoded in the DNA get to the site of protein synthesis? That’s where RNA comes into this three-player act.
That’s known as the central dogma of molecular biology. It states that “DNA makes RNA makes protein.” This process starts with DNA. And first DNA had to be identified as the genetic material.
For almost 100 years, scientists have known plenty about proteins. They have known that proteins of all different shapes, sizes, and functions exist. For this reason, many scientists believed that proteins were the heredity material. It wasn’t until 1928, when Frederick Griffith identified the process of transformation, that individuals started to question this concept. Griffith demonstrated that transformation occurs, but what was the material that caused the transforming process?
Griffith was studying Streptococcus pneumoniae, a bacterium that infects mammals. He used two strains, a virulent S (smooth) strain and a harmless R (rough) strain to demonstrate the transfer of genetic material. The S strain is surrounded by a polysaccharide capsule, which protects it from the host’s immune system, resulting in the death of the host, while the R strain, which does not have the protective capsule, is defeated by the host’s immune system. Hence, when mammalian cells are infected with the R strain bacteria, the host does not die (Figure 8.1).
Griffith infected mice with heat-killed S strain bacteria. As expected, the heat-killed bacteria, as they were dead, had no effect on the mice (Figure 1). But then he tried something different.
www.ck12.org 348
He mixed the remains of heat-killed S strain bacteria with live R strain bacteria and injected the mixture into mice. Remember, separately both of these bacteria are harmless to the mice. And yet the mice died (Figure 8.1). Why? These mice had both live R and live S strain bacteria in their blood. How? Griffith concluded that the R strain had changed, or transformed, into the lethal S strain. Something, such as the ”instructions” from the remains of the S strain, had to move into the R strain in order to turn the harmless R strain into the lethal S strain. This material that was transferred between strains had to be the heredity material. But the transforming material had yet to be identified. Transformation is now known as the change in genotype and phenotype due to the assimilation of external DNA (heredity material) by a cell.
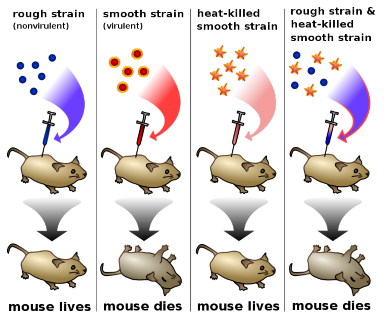
Over the next decade, scientists, led by Oswald Avery, tried to identify the material involved in transformation. Avery, together with his colleagues Maclyn McCarty and Colin MacLeod, removed various organic compounds from bacteria and tested the remaining compounds for the ability to cause transformation. If the remaining material did not cause transformation, than that material could not be the heredity material. Avery treated S strain bacteria with protease enzymes, which remove proteins from cells, and then mixed the remainder with R strain bacteria. The R strain bacteria transformed, meaning that proteins did not carry the genes for causing the disease. Then the remnants of the S strain bacteria were treated with deoxyribonuclease, an enzyme which degrades DNA. After this treatment, the R strain
bacteria no longer transformed. This indicated that DNA was the heredity material. The year was 1944.
However, this finding was not widely accepted, partly because so little was known about DNA. It was still thought that proteins were better candidates to be the heredity material. The structure of DNA was still unknown, and many scientists were not convinced that genes from bacteria and more complex organisms could be similar.
In 1952, Alfred Hershey and Martha Chase put this skepticism to rest. They conclusively demonstrated that DNA is the genetic material. Hershey and Chase used the T2 bacte- riophage, a virus that infects bacteria, to prove this point. A virus is essentially DNA (or RNA) surrounded by a protein coat (Figure 8.2). To reproduce, a virus must infect a cell and use that host cell’s machinery to make more viruses. The T2 bacteriophage can quickly turn an Escherichia coli (E. coli) bacteria into a T2 producing system. But to do that, the genetic material from T2, which could only be protein or DNA, must be transferred to the bacteria. Which one was it?
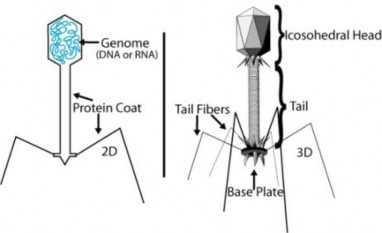
Hershey and Chase performed a series of classic experiments, taking advantage of the fact that T2 is essentially just DNA and protein. In the experiments, T2 phages with either radioactive 32P-labeled DNA or radioactive 35S-labeled protein were used to infect bacteria. Either the radioactive proteins or radioactive DNA would be transferred to the bacteria. Identifying which one is transferred would identify the genetic material. In both experiments, bacteria were separated from the phage coats by blending, followed by centrifugation. Only the radioactively labeled DNA was found inside the bacteria, whereas the radioactive proteins stayed in the solution (Figure 8.3). These experiments demonstrated that DNA is the genetic material and that protein does not transmit genetic information.
www.ck12.org 350
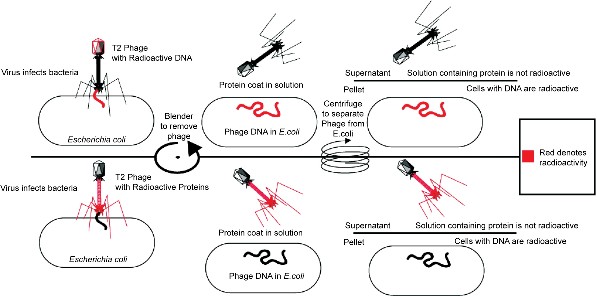
It was known that DNA is composed of nucleotides, each of which contains a nitrogen- containing base, a five-carbon sugar (deoxyribose), and a phosphate group. In these nu- cleotides, there is one of the four possible bases: adenine (A), guanine (G), cytosine (C), or thymine (T) (Figure 8.4).
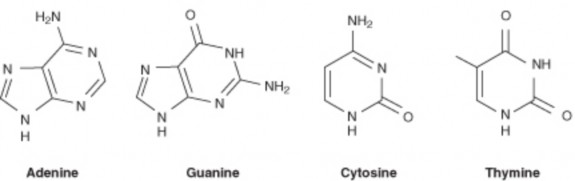
Figure 8.4: Chemical structure of the four nitrogenous bases in DNA. (13)
Erwin Chargaff proposed two main rules that have been appropriately named Chargaff’s rules. In 1947 he showed that the composition of DNA varied from one species to another. This molecular diversity added evidence that DNA could be the genetic material. Chargaff also determined that in DNA, the amount of one base always approximately equals the amount of a particular second base. For example, the number of guanines equals the number of cytosines, and the number of adenines equals the number of thymines. Human DNA is 30.9% A and 29.4% T, 19.9% G and 19.8% C. This finding, together with that of the DNA structure, led to the base-pairing rules of DNA.
In the early 1950s, Rosalind Franklin started working on understanding the structure of DNA fibers. Franklin, together with Maurice Wilkins, used her expertise in x-ray diffraction photographic techniques to analyze the structure of DNA. In February 1953, Francis Crick and James D. Watson of the Cavendish Laboratory in Cambridge University had started to build a model of DNA. Watson and Crick indirectly obtained Franklin’s DNA X-ray diffraction data demonstrating crucial information into the DNA structure. Francis Crick and James Watson (Figure 8.5) then published their double helical model of DNA in Nature on April 25th, 1953.
www.ck12.org 352

Figure 8.5: James Watson (left) and Francis Crick (right). (14)
DNA has the shape of a double helix, just like a spiral staircase (Figure 8.6). There are two sides, called the sugar-phosphate backbone, because they are made from alternating phosphate groups and deoxyribose sugars. The “steps” of the double helix are made from the base pairs formed between the nitrogenous bases. The DNA double helix is held together by hydrogen bonds between the bases attached to the two strands.
The double helical nature of DNA, together with the findings of Chargaff, demonstrated the base-pairing nature of the bases. Adenine always pairs with thymine, and guanine always pairs with cytosine (Figure 8.7). Because of this complementary nature of DNA, the bases on one strand determine the bases on the other strand. These complementary base pairs explain why the amounts of guanine and cytosine are present in equal amounts, as are the amounts of adenine and thymine. Adenine and guanine are known as purines. These bases consist of two ring structures. Purines make up one of the two groups of nitrogenous bases. Thymine and cytosine are pyrimidines, which have just one ring structure. By having a purine always combine with a pyrimidine in the DNA double helix, the distance between the two sugar-phosphate backbones is constant, maintaining the uniform shape of the DNA molecule.
The two strands in the DNA backbone run in anti-parallel directions to each other. That is, one of the DNA strands is built in the 5’ → 3’ direction, while the complementary strand is built in the 3’ → 5’ direction. In the DNA backbone, the sugars are joined together by phosphate groups that form bonds between the third and fifth carbon atoms of adjacent sugars. In a double helix, the direction of the nucleotides in one strand is opposite to their direction in the other strand. 5’ and 3’ each mark one end of a strand. A strand running in the 5’→ 3’ direction that has adenine will pair with base thymine on the complementary
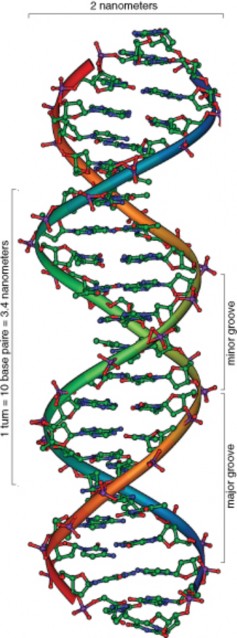
www.ck12.org 354
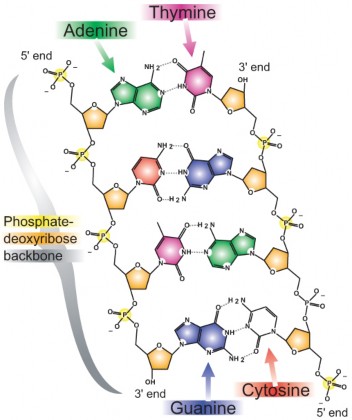
strand running in 3’→ 5’ direction.
So it is this four letter code, made of just A, C, G, and T, that determines what the organism will become and what it will look like. How can these four bases carry so much information? This information results from the order of these four bases in the chromosomes. This sequence carries the unique genetic information for each species and each individual. Humans have about 3,000,000,000 bits of this information in each cell. A gorilla may also have close to that amount of information, but a slightly different sequence. For example, the sequence AGGTTTACCA will have different information than CAAGGGATTA. The closer the evolutionary relationship is between two species, the more similar their DNA sequences will be. For example, the DNA sequences between two species of reptiles will be more similar than between a reptile and an elm tree.
DNA sequences can be used for scientific, medical, and forensic purposes. DNA sequences can be used to establish evolutionary relationships between species, to determine a person’s susceptibility to inherit or develop a certain disease, or to identify crime suspects or victims. Of course, DNA analysis can be used for other purposes as well. So why is DNA so useful for these purposes? It is useful because every cell in an organism has the same DNA sequence. For this to occur, each cell must have a mechanism to copy its entire DNA. How can so much information be exactly copied in such a small amount of time?
DNA replication is the process in which a cell’s entire DNA is copied, or replicated. This process occurs during the Synthesis (S) phase of the eukaryotic cell cycle. As each DNA strand has the same genetic information, both strands of the double helix can serve as templates for the reproduction of a new strand. The two resulting double helices are identical to the initial double helix. For an animation of DNA replication, see http://www. hhmi.org/biointeractive/media/DNAi_replication_vo1-sm.mov.
DNA replication begins as an enzyme, DNA helicase, breaks the hydrogen bonds holding the two strands together and forms a replication fork. The resulting structure has two branching strands of DNA backbone with exposed bases. These exposed bases allow the DNA to be “read” by another enzyme, DNA polymerase, which then builds the complementary DNA strand. As DNA helicase continues to open the double helix, the replication fork grows.
The two new strands of DNA are “built” in opposite directions, through either a leading strand or a lagging strand. The leading strand is the DNA strand that DNA polymerase constructs in the 5’ → 3’ direction. This strand of DNA is made in a continuous manner,
www.ck12.org 356
moving as the replication fork grows. The lagging strand is the DNA strand at the opposite side of the replication fork from the leading strand. It goes in the opposite direction, from 3’ to 5’. DNA polymerase cannot build a strand in the 3’ → 5’ direction. Thus, this “lagging” strand is synthesized in short segments known as Okazaki fragments. On the lagging strand, an enzyme known as primase builds a short RNA primer. DNA polymerase is then able to use the free 3’ OH group on the RNA primer to make DNA in the 5’ → 3’ direction. The RNA fragments are then degraded and new DNA nucleotides are added to fill the gaps where the RNA was present. Another enzyme, DNA ligase, is then able to attach (ligate) the DNA nucleotides together, completing the synthesis of the lagging strand (Figure 8.8).
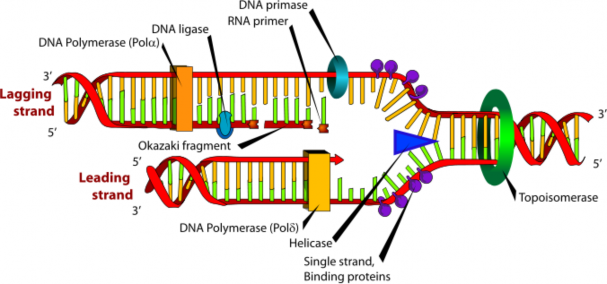
Many replication forks develop along a chromosome. This process continues until the replica- tion forks meet, and the all of the DNA in a chromosome has been copied. Each new strand that has formed is complementary to the strand used as the template. Each resulting DNA molecule is identical to the original DNA molecule. During prophase of mitosis or prophase I of meiosis, these molecules of DNA condense into a chromosome made of two identical chromatids. This process ensures that cells that result from cell division have identical sets of genetic material, and that the DNA is an exact copy of the parent cell’s DNA.
“DNA makes RNA makes protein.” So what exactly is RNA? Ribonucleic acid, or RNA, is the other important nucleic acid in the three player act. When we say that “DNA makes RNA makes protein,” what do we mean? We mean that the information in DNA is somehow transferred into RNA, and that the information in RNA is then used to make the protein.
To understand this, it helps to first understand RNA. If you remember from the chapter titled Cell Division and Reproduction, a gene is a segment of DNA that contains the information necessary to encode an RNA molecule or a protein. Keep in mind that even though you have many thousands of genes, not all are used in every cell type. In fact, probably only a few thousand are used in a particular type of cell, with different cell types using different genes. However, while these genes are embedded in the large chromosomes that never leave the nucleus, the RNA is relatively small and is able to carry information out of the nucleus.
RNA structure differs from DNA in three specific ways. Both are nucleic acids and made out of nucleotides; however, RNA is single stranded while DNA is double stranded. RNA contains the 5-carbon sugar ribose, whereas in DNA, the sugar is deoxyribose. Though both RNA and DNA contain the nitrogenous bases adenine, guanine and cytosine, RNA contains the nitrogenous base uracil instead of thymine. Uracil pairs with adenine in RNA, just as thymine pairs with adenine in DNA. A comparison of RNA and DNA is shown in Table 8.1 and Figure 8.9.
![]()
RNA DNA
![]()
single stranded double stranded
Specific Base contains uracil contains thymine
Sugar ribose deoxyribose
Size relatively small big (chromosomes)
Location moves to cytoplasm stays in nucleus
Types 3 types: mRNA, tRNA, rRNA
generally 1 type
![]()
www.ck12.org 358
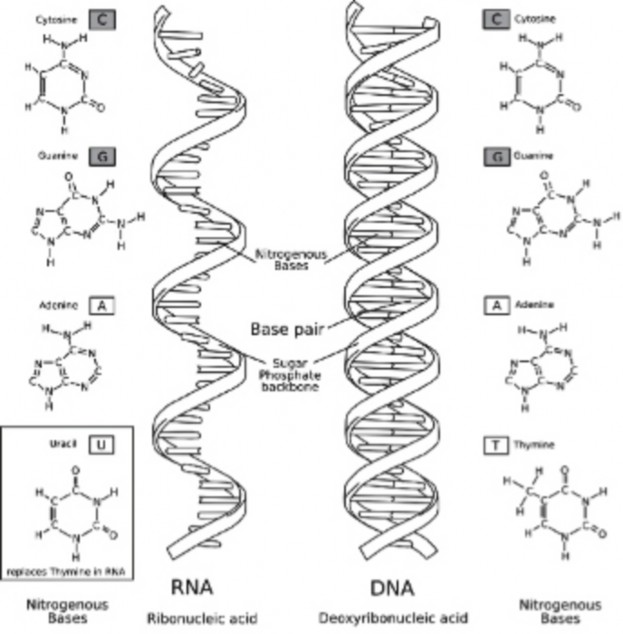
So what does RNA do? There are three types of RNA: messenger RNA (mRNA), transfer RNA (tRNA), and ribisomal RNA (rRNA). All three of these nucleic acids work together to produce a protein. The mRNA takes the instructions from the nucleus to the cytoplasm, where the ribosomes are located. Ribosomes are where the proteins are made. The ri- bosomes themselves are made out of rRNA and other proteins. The mRNA binds to the ribosome, bringing the instructions to order the amino acids to the site of protein synthesis. Finally, the tRNA brings the correct amino acid to the site of protein synthesis (Figure
and Figure 8.11). In mRNA, the four nucleotides (A, C, G, and U) are arranged into codons of three bases each. Each codon encodes for a specific amino acid, except for the stop codons, which terminate protein synthesis. tRNA, which has a specific “3-leaf clover structure,” contains a three base region called the anticodon, which can base pair to the corresponding three-base codon region on mRNA. More will be discussed on these processes during the lesson on translation that follows.
Remember, proteins are made out of amino acids, so how does the information get converted from the language of nucleotides to the language of amino acids? The process is called translation.
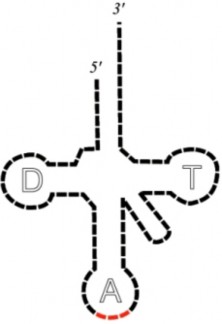
www.ck12.org 360
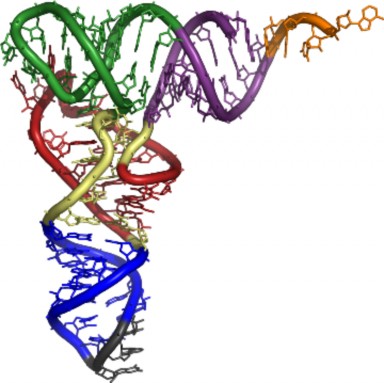
Small interfering RNA (siRNA), microRNA (miRNA) and small nuclear RNA (snRNA): siRNA and miRNA are revolutionizing molecular biology, developmental biology, and even medicine. The 2006 Nobel prize in Physiology and Medicine was awarded to Dr. Andrew Fire and Dr. Craig Mello for their discovery of siRNA, which is a type of double-stranded RNA that inhibits gene expression at the mRNA level. Specifically, siRNA acts on ho- mologous processed mRNA by targeting it for degradation. siRNA is responsible for RNA interference (RNAi). RNAi has a natural role in that it is used by plants in defense against plant viral RNAs. miRNAs are also involved in the regulation of gene expression. They are transcribed but not translated into proteins. snRNAs are found within the nucleus of eukaryotic cells. They are involved in a variety of important processes such as RNA splic- ing (removal of introns), and regulation of transcription factors (discussed in Lesson 8.2: Protein Synthesis).
Griffith demonstrated the process of transformation, which is the change in genotype and phenotype due to the assimilation of the external DNA by a cell.
Avery and colleagues demonstrated that DNA was the transforming material.
The Hershey and Chase experiments conclusively demonstrated that DNA is the ge- netic material.
Watson and Crick demonstrated the double helix model of DNA.
The Base paring rules state that A always pairs with T and G always pairs with C.
DNA replication is the process by which a cell’s entire DNA is copied, or replicated.
During DNA replication, the two new strands of DNA are “built” in opposite directions, starting at replication forks.
RNA is a single-stranded nucleic acid.
RNA contains the nitrogenous base uracil.
There are three types of RNA: mRNA, tRNA, and rRNA.
mRNA is the intermediary between the nucleus, where the DNA lives, and the cyto- plasm, where proteins are made.
Discuss how DNA was identified as the genetic material.
Define transformation.
In DNA, why does the amount of adenine approximately equal the amount of thymine?
What are the base pairing rules?
Explain Watson and Crick’s double helix model of DNA.
How is DNA replicated?
Discuss the importance of mRNA.
Explain the main differences between mRNA, rRNA,and tRNA. www.ck12.org 362
Campbell, N.A. and Reece, J.B. Biology, Seventh Edition, Benjamin Cummings, San Francisco, CA, 2005.
Biggs, A., Hagins, W.C., Kapicka, C., Lundgren, L., Rillero, P., Tallman, K.G., and Zike, D., Biology: The Dynamics of Life, California Edition, Glencoe Science, Colum- bus, OH, 2005.
Nowicki S., Biology, McDougal Littell, Evanston, IL, 2008. The Dolan DNA Learning Center.
DNA Interactive:
A Science Odyssey:
National Human Genome Research Institute:
The RNA Modification Database:
The RNA World:
http://nobelprize.org/nobel_prizes/chemistry/articles/altman/index.html
amino acid The monomers that combine to form a polypeptide (protein).
anticodon A 3 base sequence on the tRNA that base pairs with the codon on the mRNA.
anti-parallel Describes the orientation of the two DNA strands; one of the DNA strands is built in the 5’ → 3’ direction, while the complementary strand is built in the 3’ → 5’ direction.
bacteriophage A virus that infects bacteria.
codon A sequence of three nucleotides within mRNA; encodes for a specific amino acid or termination (stop) sequence.
deoxyribonuclease An enzyme which degrades DNA.
DNA Deoxyribonucleic acid, the genetic (heredity) material.
DNA polymerase The enzyme that builds a new DNA strand during DNA replication.
DNA helicase The enzyme that breaks the hydrogen bonds holding the two DNA strands together during DNA replication.
DNA ligase An enzyme that joins broken nucleotides together by catalyzing the formation of a bond between the phosphate group and deoxyribose sugar of adjacent nucleotides in the DNA backbone.
DNA replication The process in which a cell’s entire DNA is copied.
double helix The shape of DNA, resembling a spiral staircase.
gene A segment of DNA that contains the information necessary to encode an RNA molecule or a protein.
lagging strand The DNA strand at the opposite side of the replication fork from the leading strand.
leading strand The DNA strand that DNA polymerase constructs in the 5’ → 3’ direction.
mRNA Messenger RNA; serves as a nucleic acid intermediate between the nucleus and the ribosomes.
nucleotide Monomer of nucleic acids, composed of a nitrogen-containing base, a five- carbon sugar, and a phosphate group.
Okazaki fragments Short fragments of DNA that comprise the lagging strand.
primase An enzyme that builds a short RNA primer on the lagging strand during DNA replication.
purines Nitrogenous bases consisting of two ring structures; adenine and guanine. pyrimidines Nitrogenous bases consisting of one ring structure; thymine and cytosine. ribosome Non-membrane bound organelle; site of protein synthesis.
www.ck12.org 364
RNA Ribonucleic acid; single-stranded nucleic acid.
rRNA Ribosomal RNA; together with proteins, forms ribosomes.
sugar-phosphate backbone The sides of the DNA double helix; composed of alternating phosphate groups and deoxyribose sugars.
transcription The process of making an mRNA from the information in the DNA se- quence.
translation The process of making a protein from the information in a mRNA sequence.
transformation The change in genotype and phenotype due to the assimilation of external DNA (heredity material) by a cell.
tRNA Transfer RNA; brings amino acids to the ribosome.
”DNA → RNA” Can you think of a method in which the information in DNA is transferred to an RNA molecule?
Can you hypothesize on how the As, Cs, Gs and Us of RNA can code for the 20 amino acids of proteins?
Can you develop a model in which the three types of RNAs interact to make a protein?
Discuss the meaning of DNA → RNA → Protein.
Describe how transcription makes RNA from a DNA template.
Explain the various types of modification mRNA undergoes before translation.
Discuss mRNA splicing and define introns and exons.
Explain how the Genetic Code is a three letter code, and describe its role in translating nucleotides into amino acids.
Explain that a reading frame is the group of three bases in which the mRNA is read, and describe how interrupting the reading frame may have severe consequences on the protein.
Discuss what is meant by the universal genetic code.
Describe translation. Explain that translation is the process of ordering the amino acids into a polypeptide; translation involves changing the language of nucleotides into the language of amino acids.
Illustrate the process of translation, describing how mRNA, rRNA, and tRNA all work together to complete the process.
Discuss what happens to the polypeptide after translation.
The central dogma of molecular biology describes the fundamental process that makes us all different. We all have different proteins. That is, though they may be the same types of proteins, such as we have the protein collagen found in bones, many of our proteins are slightly different and thus work slightly differently. If all our proteins acted the same way, we would all be exactly the same. But because we all have different DNA sequences, and DNA contains genes, and genes contain the information to encode an RNA molecule or a protein, we are all different.
So how does “DNA makes RNA makes protein” actually happen? The two processes nec- essary to make a protein from the information in DNA are transcription and translation. Transcription, which happens in the nucleus, uses the DNA sequence to make an RNA molecule. The RNA then leaves the nucleus and goes to the cytoplasm where translation occurs on a ribosome and produces a protein.
Transcription is “DNA → RNA.” In other words, transcription is the transfer of the ge- netic “instructions” from DNA to RNA. During transcription, a complementary copy of RNA is made. Whereas in DNA replication both strands of the DNA double helix are used as templates, in transcription only one strand is needed. RNA polymerase enzy- matically “reads” a template strand of DNA, known as the coding strand, to synthesize the complementary RNA strand. Transcription is divided into 3 stages, appropriately named initiation, elongation and termination. For an animation of transcription, see http:
//www.hhmi.org/biointeractive/media/DNAi_transcription_vo1-sm.mov.
Transcription begins with the binding of RNA polymerase to the promoter of a gene. An eukaryotic promoter usually includes specific sequences that are recognized by transcription factors, which are proteins that aid in the binding of RNA polymerase to the correct place on the DNA. The transcription initiation complex formed by the promoter, transcription factors, and RNA polymerase signals the start, or initiation, of transcription. The DNA
www.ck12.org 366
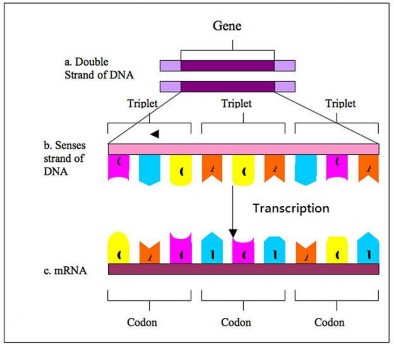
unwinds and produces a small open complex, which allows RNA polymerase to “read” the DNA template and begin the synthesis of RNA.
Transcription elongation involves the further addition of RNA nucleotides and the change of the open complex to a transcriptional complex. As the RNA transcript is assembled, DNA in front of RNA polymerase unwinds and transcription continues. As transcription progresses, RNA nucleotides are added to the 3’ end of the growing RNA transcript. The transcriptional complex has a short DNA-RNA hybrid, an 8 base-pair stretch in which the newly made RNA is temporarily hydrogen bonded to the DNA template strand. Unlike DNA replication, mRNA transcription can involve multiple RNA polymerases, allowing numerous mRNAs to be produced from a single copy of the gene. This step also involves a proofreading mechanism that can replace an incorrectly added RNA nucleotide.
The termination of transcription in prokaryotes and eukaryotes is very different. Though both involve the detachment of the RNA from the DNA template, how this occurs is sur- prisingly distinct. Bacteria use two different strategies for transcription termination, Rho- dependent and Rho-independent termination. In Rho-dependent termination, a pro- tein factor called ”Rho” destabilizes the RNA-DNA hybrid, releasing the newly synthesized mRNA from the elongation complex. In Rho-independent termination, RNA transcription stops when the newly synthesized RNA molecule forms a hairpin loop followed by a run of uracils. This structure is the signal for the detachment of the RNA from the DNA. The DNA is now ready for translation.
The termination of transcription in eukaryotes is less well understood. The RNA poly- merase transcribes a polyadenylation signal. Polyadenylation is the addition of a string of A’s to the mRNA’s 3’ end and will be discussed in the next section. However, soon after the transcription of this signal, proteins cut the RNA transcript free from the polymerase and the polymerase eventually falls off the DNA. This process produces a pre-mRNA, an mRNA that is not quite ready to be translated.
Newly transcribed eukaryotic mRNA is not ready for translation. This mRNA requires extensive processing, and so is known as pre-mRNA. The modification processes include splicing, the addition of a 5’ cap, editing, and polyadenylation. Once these process have occurred, the mature mRNA can be exported through the nuclear pore.
www.ck12.org 368
Humans have approximately 22,000 genes, yet make many more proteins. How? A process called alternative splicing allows one mRNA to produce many polypeptides. To under- stand this concept, the structure of the pre-mRNA must be discussed.
Eukaryotic pre-mRNA contains introns and exons. An exon is the region of a gene that contains the code for producing a protein. Most genes contain many exons, with each exon containing the information for a specific portion of a complete protein. In many species, a gene’s exons are separated by long regions of DNA that have no identified function. These long regions are introns, and must be removed prior to translation. Splicing is the process by which introns are removed (Figure 8.13). Sometimes a process called alternative splicing allows pre-mRNA messages to be spliced in several different configurations, allowing a single gene to encode multiple proteins. Splicing is usually performed by an RNA-protein complex called the spliceosome, but some RNA molecules have their own catalytic activity and are capable of acting like enzymes to catalyze their own splicing. For an animation of RNA splicing, see http://vcell.ndsu.edu/animations/mrnasplicing/first.htm.
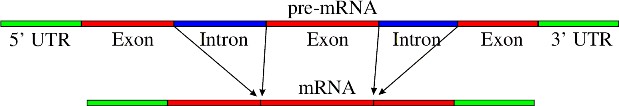
How does the mRNA know it is time to leave the nucleus? Once the mRNA leaves the nucleus, how does it find a ribosome? A signal on the front, 5’-end of the mRNA helps with both jobs. This signal is the 5’ cap. The 5’ cap is a modified guanine nucleotide added to the 5’-end of the pre-mRNA. This 5’ cap is crucial for recognition and proper attachment of the mRNA to the ribosome, as well as protection from exonucleases, enzymes that degrade nucleic acids.
In certain instances, the nucleotide sequence of an mRNA will be changed to allow the mRNA to produce multiple proteins. This process is called editing. The classic example is editing
of the apolipoprotein B (APOB) mRNA in humans. The APOB protein occurs in the plasma in two main forms, APOB48 and APOB100. The first is synthesized exclusively by the small intestine, the second by the liver. Both proteins are coded for by the same gene, which is transcribed into a single pre-mRNA. Editing creates a premature (early) stop codon, which upon translation produces a smaller protein. As a result of the RNA editing, APOB48 and APOB100 share a common N-terminal sequence, but APOB48 lacks APOB100’s C-terminal region.
In eukaryotic cells, the transcription of the polyadenylation signals indicates the termination of the process. The mRNA transcript is then cut off of the RNA polymerase and freed from the DNA. The cleavage site is characterized by the presence of the sequence AAUAAA near the end of the transcribed message. Polyadenylation then occurs. Polyadenylation is the addition of a poly(A) tail to the 3’-end of the mRNA. The poly(A) tail may consist of as many as 80 to 250 adenosine residues. The poly(A) tail protects the mRNA from degradation by exonucleases. Poladenylation is also important for transcription termination, export of the mRNA from the nucleus, and translation. For an animation on RNA polyadenylation, see http://vcell.ndsu.edu/animations/mrnaprocessing/first.htm.
So how exactly is the language of nucleotides used to code for the language of amino acids? How can a code of only As, Cs, Gs, and Us carry information for 20 different amino acids? The genetic code is the code in which the language of nucleotides is used to create the language of amino acids.
A code of at least three letters has to be the answer. A one letter code would only be able to code for four amino acids. A two letter code could only code for 16 amino acids. With a three letter code, there are 64 possibilities. As there are 20 amino acids, the answer must be a code of at least three letters.
In 1961, Francis Crick and Sydney Brenner demonstrated the presence of codons, that is, three bases of RNA that code for one amino acid (Figure 8.14). Also in 1961, Marshall Nirenberg and Heinrich Matthaei at the National Institutes of Health (NIH) demonstrated that a poly(U) RNA sequence was translated into a polypeptide consisting of only the amino acid phenylalanine. This proved that the codon UUU coded for the amino acid phenylalanine. Extending this work, Nirenberg and his coworkers were able to determine the nucleotide makeup of 54 of the 64 codons. Others determined the remainder of the genetic code (Figure
www.ck12.org 370
8.15).
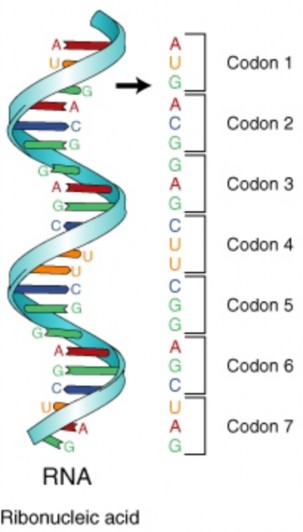
The codon AUG codes for the amino acid methionine. This codon is also the start codon which begins every translation of every amino acid chain. The translational machinery “reads” the mRNA codon by codon until it reaches a stop, or termination, codon. Stop
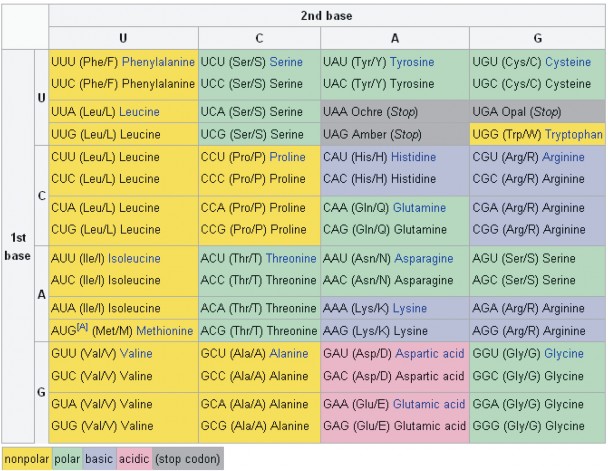
www.ck12.org 372
codons are not associated with a tRNA or amino acid. Three are three stop codons: UAG, UGA, UAA.
The reading frame is the frame of three bases in which the mRNA is read or translated. Every sequence can be read in three reading frames, each of which will produce a different amino acid sequence. For example, in the sequence GCAUGGGGGUCUAG, the reading frame can begin with either the first G, the first C, or the first A. As stated above, trans- lation starts with the start codon which consists of the three bases AUG. Each subsequent codon is translated until an in-frame stop codon is reached. In the example above, the polypeptide sequence would be: methionine – glycine – valine – stop.
Mutations that disrupt the reading frame by insertions or deletions of a non-multiple of 3 nucleotide bases are known as frameshift mutations. Take the example:
THE BIG FAT CAT ATE THE RED RAT
A deletion mutation that disrupts the reading frame, results in a message that does not make any sense. If the ‘B’ is deleted:
THE IGF ATC ATA TET HER EDR AT
Once the reading frame is disrupted, the mRNA may not be translated properly. These mutations may impair the function of the resulting protein, if the protein is even formed. Many frameshift mutations result in a premature stop codon, in other words, a stop codon that come earlier than normal during translation. This would result in a smaller protein, most likely without normal function.
When there are 64 codon combinations for 20 amino acids (and stop codons), there is going to be some overlap. Within the genetic code there is redundancy but no ambiguity. For example, the codons GGG, GGA, GGC, and GGU all encode the amino acid glycine, but none encode another amino acid. Degenerate codons often differ in the third position.
The genetic code is said to be universal. That is, the same code is utilized by the simplest prokaryotic organism and by humans. This universality is a tremendous benefit to mankind. If a human gene is placed in a bacteria, it looks just like a piece of DNA to the bacteria. The human As, Cs, Gs, and Ts look just like the bacteria’s As, Cs, Gs, and Ts. So, the bacterial proteins will transcribe and translate this DNA, making a human protein.
But how exactly are these proteins made? We have been referring to mRNA’s, tRNA’s, ribosomes, codons and the genetic code throughout this chapter. How do they all come together to make a protein? The process is called translation.
Translation is “RNA → protein.” In other words, translation is the transfer of the instructions in RNA to a protein made of amino acids. Translation uses the products of transcription, mRNA, tRNA, and rRNA, and converts the mRNA sequence into a polypeptide according to the genetic code. The mRNA moves to the cytoplasm and interacts with a ribosome, which serves as the site of translation. Translation proceeds in three phases: initiation, elongation and termination.
To understand translation, first we need to understand the ribosome. Ribosomes are com- posed of two subunits, a small subunit and a larger subunit. Prokaryotic subunits are named the 30S and 50S subunits; eukaryotic subunits are named the 40S and 60S subunits. Dur- ing translation the tRNA molecules are literally “inside” the ribosomal subunits, as they sit on the mRNA strand. When tRNAs come to the ribosome, adjacent amino acids are brought together, allowing the ribosome to catalyze the formation of the peptide bond be- tween amino acids. The ribosome has three tRNA binding sites: the A site, the P site, and the E site (Figure 8.16). The A site binds a tRNA with an attached amino acid. The P site contains the tRNA with the growing polypeptide chain attached, and the E site contains the tRNA that no longer has an attached amino acid. This tRNA is preparing to exit the ribosome. A single mRNA can be translated simultaneously by multiple ribosomes. For an animation of translation, see http://www.hhmi.org/biointeractive/media/DNAi_ translation_vo1-sm.mov.
Figure 8.16: This cartoon depicts the relative location of the E, P, and A sites within the ribosome. The A site binds a tRNA bound to an amino acid, the P site binds a tRNA bound to the polypeptide being synthesized, and the E site binds a tRNA without an attached amino acid before the tRNA exits the ribosome. (1)
The initiation of translation in prokaryotes involves the assembly of the ribosome and addi- tion of the first amino acid, methionine. The 30S ribosomal subunit attaches to the mRNA. Next, the specific methionine tRNA is brought into the P. The anticodon of this tRNA will bind to the AUG codon on the mRNA. This is the only time a tRNA will be brought into the P site; all successive tRNA’s will be brought to the A site as translation continues. The 50S ribosomal subunit then binds to the 30S subunit, completing the ribosome.
www.ck12.org 374
The initiation of protein translation in eukaryotes is similar to that of prokaryotes with some minor modifications. The 5’ cap and 3’ poly(A) tail are involved in the recruitment of the ribosome. In eukaryotes the ribosome scans along the mRNA for the first methionine codon. Translation may begin at all AUG codons, however only an in-frame AUG will produce a functional polypeptide. For an animation of translation, see http://vcell.ndsu.edu/ animations/translation/first.htm.
Elongation is fairly similar between prokaryotes and eukaryotes. As translation begins, the start tRNA is sitting on the AUG codon in the P site of the ribosome, so the next codon available to accept a tRNA is at the A site. Elongation proceeds after initiation with the binding of an tRNA to the A site. The next tRNA binds to the codon, bringing the appropriate amino acid to the ribosome, and a peptide bond joins between the start methionine and the next amino acid. The entire ribosome complex moves along the mRNA, sending the first tRNA into the E site and the tRNA with the growing polypeptide into the P site. The A site is now empty and ready to accept another tRNA. The first tRNA now leaves the ribosome. The A site accepts a tRNA with an attached amino acid, a peptide bond forms between the two adjacent amino acids, and the process continues.
Termination of translation occurs when the ribosome comes to one of the three stop codons, for which there is no tRNA. At this point, a protein called a release factor binds to the A site. The release factor causes the addition of a water molecule to the polypeptide chain, resulting in the release of the completed chain from the tRNA and ribosome. The ribosome, release factor, and tRNAs then dissociate and translation is complete. The process of translation is summarized in Figure 8.17.
The events following protein synthesis often include post-translational modification of the peptide chain and folding of the protein into its functional conformation. During and after synthesis, polypeptide chains often fold into secondary and then tertiary structures. These levels of organization were discussed in the chapter titled Chemical Basis of Life. Briefly, the primary structure of the protein is determined by the gene. The secondary and ter- tiary structures are determined by interactions between the amino acids within the protein (Figure 8.18).
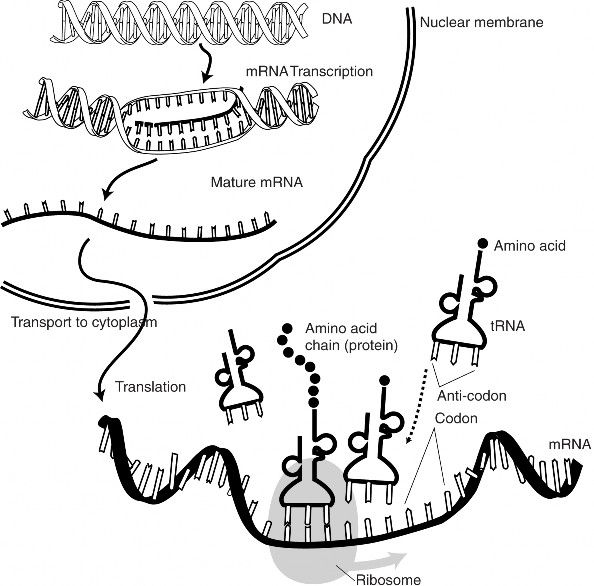
www.ck12.org 376
Many proteins undergo post-translational modification, allowing them to then perform their specific function. This may include the formation of disulfide bridges or attachment of any of a number of biochemical functional groups, such as phosphate groups, carbohydrates or lipids. Certain amino acids may be removed, or the polypeptide chain may be cut into two pieces. Lastly, two or more polypeptides may interact with each other, forming a functional protein with a quaternary structure.
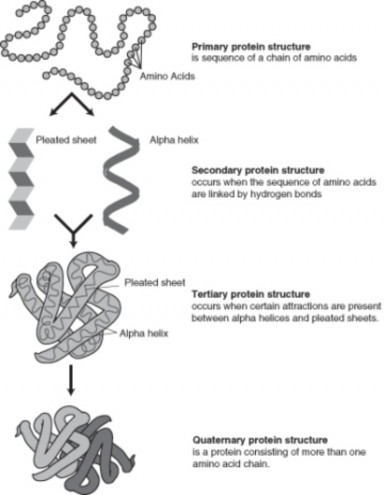
Figure 8.18: The four stages of protein folding. (19)
“DNA makes RNA makes protein” is the central dogma of molecular biology.
Transcription is the transfer of the genetic instructions from DNA to RNA.
Translation is the process of using the information in the mRNA to order amino acids into a polypeptide.
Transcription begins with the binding of RNA polymerase to the promoter of a gene.
Newly transcribed eukaryotic mRNA is not ready for translation; this mRNA requires extensive processing, including splicing and polyadenylation.
A codon is a three base code for one amino acid.
Start and stop codons signal the beginning and end of translation.
There are three stop codons.
The reading frame is the frame of three bases in which the mRNA is read.
The genetic code is universal.
Translation involves the interactions of the three types of RNA.
After the protein is made, it must fold into its functional conformation.
What is meant by “DNA → RNA → Protein?”
Describe transcription.
List some of the modification mRNAs undergoes before translation.
Define introns and exons.
Describe mRNA splicing.
Describe the role of the Genetic Code in translation.
What is a reading frame?
Discuss what is meant by the universal genetic code.
Explain translation.
What is protein folding?
Transcription Animation
http://www-class.unl.edu/biochem/gp2/m_biology/animation/gene/gene_a2.html
Campbell, N.A. and Reece, J.B. Biology, Seventh Edition, Benjamin Cummings, San Francisco, CA, 2005.
Biggs, A., Hagins, W.C., Kapicka, C., Lundgren, L., Rillero, P., Tallman, K.G., and Zike, D., Biology: The Dynamics of Life, California Edition, Glencoe Science, Colum- bus, OH, 2005.
Nowicki S., Biology, McDougal Littell, Evanston, IL, 2008.
The National Health Museum:
Genetic Science Learning Center: www.ck12.org 378
http://learn.genetics.utah.edu/units/basics/transcribe/
Kimball’s Biology Pages:
http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/C/Codons.html
The National Health Museum:
http://www.accessexcellence.org/RC/VL/GG/genetic.html
The National Health Museum:
http://www.accessexcellence.org/RC/VL/GG/protein_synthesis.html
5’ cap A modified guanine nucleotide added to the 5’-end of the pre-mRNA; crucial for recognition and proper attachment of the mRNA to the ribosome.
A site Within the ribosome; binds a tRNA with an attached amino acid.
alternative splicing Process by which pre-mRNA messages can be spliced in several dif- ferent configurations, allowing a single gene to encode multiple proteins.
E site Within the ribosome; contains the tRNA that no longer has an attached amino acid.
editing The process of changing the nucleotide sequence of an mRNA to allow the mRNA to produce multiple proteins.
elongation The segment of transcription that the further addition of RNA nucleotides; also refers to the process during translation of adding additional amino acids to the growing polypeptide chain.
exon The region of a gene that contains the code for producing a protein.
frameshift mutation Mutation that disrupt the reading frame by insertions or deletions of a non-multiple of three nucleotide bases.
gene A segment of DNA that contains the information necessary to encode an RNA molecule or a protein.
genetic code The code in which the language of nucleotides is used to create the language of amino acids.
initiation The start of transcription; signaled by the transcription initiation complex formed by the promoter, transcription factors, and RNA polymerase; also refers to the start of translation.
intron Long region of DNA that has no identified function; separates exons.
P site Within the ribosome; contains the tRNA with the growing polypeptide chain at- tached.
pre-mRNA Newly transcribed eukaryotic mRNA; not yet ready for translation.
polyadenylation The addition of a string of adenines to the mRNAs 3’-end; signals the termination of translation in eukaryotes.
reading frame The frame of three bases (codon) in which the mRNA is translated.
Rho-dependent termination Involves a protein factor (Rho), which destabilizes the RNA-DNA hybrid, releasing the newly synthesized mRNA from the elongation com- plex.
ribosome Non-membrane bound organelle; site of protein synthesis.
RNA polymerase The enzyme that reads a template strand of DNA during transcription to synthesize the complementary RNA strand.
splicing The process by which introns are removed from pre-mRNA.
spliceosome RNA-protein complex that usually performs splicing of the pre-mRNA.
termination The end of transcription; involves the detachment of the RNA from the DNA template; also refers to the end of translation when the ribosome comes to one of the three stop codons, for which there is no tRNA.
transcription The process that uses the DNA sequence to make an mRNA molecule.
translation The process of converting the language of nucleotides (in the mRNA) into the language of amino acids.
www.ck12.org 380
We know what happens when everything goes right. The result is a correctly made protein that functions properly and maintains homeostasis.
However, what happens when things do not go right? What can lead to a protein not being made correctly or not functioning correctly?
Can you think of possible mechanisms that may that can interfere with protein syn- thesis?
How can a change in the DNA sequence lead to a different protein?
Define mutation.
Describe common causes of mutation.
Describe common types of mutation.
Illustrate common chromosomal alterations.
Discuss potential outcomes of point mutations.
List and describe three common types of point mutations.
Discuss consequences of effect-on-function mutations.
Discuss the significance of germline and somatic mutations.
Explain why some mutations are harmful and some beneficial.
Discuss the saying, “Without beneficial mutations, evolution can not occur.”
You have learned that an allele is an alternative form of a gene. Most, if not all genes have alternative forms causing the resulting protein to function slightly differently. But are there alleles that cause proteins to function dramatically differently or not function at all? A mutation is a change in the DNA or RNA sequence, and many mutations result in new alleles. Some of these changes can be beneficial. In fact, evolution could not take place without the genetic variation that results from mutations. But some mutations are harmful. There are also chromosomal mutations, large changes with dramatic effects.
Is it possible for mutations to occur spontaneously, or does there have to be a cause of the mutation? Well, the answer is that both are possible. A spontaneous mutation can just
happen, possibly due to a mistake during DNA replication or transcription. Mutations may also occur during mitosis and meiosis. A mutation caused by an environmental factor, or mutagen, is known as an induced mutation. Typical mutagens include chemicals, like those inhaled while smoking, and radiation, such as X-rays, ultraviolet light, and nuclear radiation. Table 8.2 lists some spontaneous mutations that are common.
Table 8.2: Common Spontaneous Mutations
![]()
Tautomerism a base is changed by the repositioning of a hydrogen atom
![]()
Depurination loss of a purine base (A or G)
Deamination spontaneous deamination of 5-
methycytosine
Transition a purine to purine, or a pyrimidine to pyrim- idine change
Transversion a purine becomes a pyrimidine, or vice versa
![]()
In multicellular organisms, mutations can be subdivided into germline mutations, which can be passed on to descendants, and somatic mutations, which cannot be transmitted to the next generation. Germline mutations change the DNA sequence within a sperm or egg cell, and therefore can be inherited. This inherited mutation may result in a class of diseases known as a genetic disease. The mutation may lead to a nonfunctional protein, and the embryo may not develop properly or survive. Somatic mutations may affect the proper functioning of the cell with the mutation. During DNA replication, the mutation will be copied. The two daughter cells formed after cell division will both carry the mutation. This may lead to the development of many cells that do not function optimally, resulting a less than optimal phenotype. Various types of mutations can all have severe effects on the individual. These include point mutations, framehift mutations and chromosomal alterations.
Keep in mind, some mutations may be beneficial or have no effect. Mutations that have no effect will not affect the expression of the gene or the sequence of amino acids in an encoded protein.
Chromosomal alterations are large changes in the chromosome structure. They occur when a section of a chromosome breaks and rejoins incorrectly, or does not rejoin at all. Sometimes the segment may join backwards or reattach to another chromosome altogether. These mutations are very serious and usually lethal to the zygote or embryo. If the embryo
www.ck12.org 382
does survive, the resulting organism is usually sterile and thus, unable to pass along the mutation.
The five types of chromosomal alterations are deletions, duplications, insertions, inversions, and translocations (Figure 8.19).
Deletions: removal of a large chromosomal region, leading to loss of the genes within that region.
Duplications (or amplifications): lead to multiple copies of a chromosomal region, increasing the number of the genes located within that region. Some genes may be duplicated in their entirety.
Insertions: the addition of material from one chromosome to a nonhomologous chro- mosome.
Inversions: reversing the orientation of a chromosomal segment.
Translocations: interchange of genetic material between nonhomologous chromo- somes.
As the name implies, point mutations occur at a single site within the DNA. Lets go back to our earlier example from lesson 8.2:
THE BIG FAT CAT ATE THE RED RAT.
A change at any one position could result in a sequence that does not make sense. Such as: THE BIG FAT SAT ATE THE RED RAT.
As shown above, point mutations exchange one nucleotide for another and are known as base substitution mutations. These mutations are often caused either by chemicals or by a mistake during DNA replication. A transition exchanges a purine for a purine (A G) or a pyrimidine for a pyrimidine, (C T), and is the most common point mutation. Less common is a transversion, which exchanges a purine for a pyrimidine or a pyrimidine for a purine (C/T A/G). Point mutations that occur within the protein coding region of a gene are classified by the effect on the resulting protein:
Silent mutations: which code for the same amino acid.
Missense mutations: which code for a different amino acid.
Nonsense mutations: which code for a premature stop codon.
These mutations may result in a protein with the same function, with altered function, or with no function. Silent mutations, as they code for the same amino acid, will have no altered effect on the protein. Missense mutations may have a minor effect or a dramatic
www.ck12.org 384
effect on the protein. Nonsense mutations usually have the most dramatic effet. Depending on the position of the premature stop codon, nonsense mutations may result in an unstable mRNA that cannot be translated, or in a much ”smaller” protein without any activity.
As pointed out earlier, a deletion or insertion in the DNA can alter the reading frame. Deletions remove one or more nucleotides from the DNA, whereas insertions add one or more nucleotides into the DNA. These mutations in the coding region of a gene may also alter splicing of the mRNA (splice site mutation). Mutations which alter the reading frame are known as frameshift mutations. Splice site mutations and frameshift mutations both can dramatically change the mRNA, altering the final protein product.
For a cell or organism to maintain homeostasis, the proteins work in a highly defined and regulated manner. It may take just one protein not working correctly to interrupt homeosta- sis. A protein having more or less activity than normal, or a different activity or function, may be enough to interrupt homeostasis. Mutations that may result in altered function of the gene product or protein are loss-of-function and gain-of-function mutations, as well as dominant negative mutations.
Loss-of-function mutations result in a gene product or protein having less or no function. Gain-of-function mutations result in the gene product or protein having a new and abnormal function. Dominant negative mutations have an altered gene product that acts in a dominant manner to the wild-type gene product.
Imagine the coding sequence (broken up into codons) TAC CCC GGG. This is a fairly generic coding sequence. It transcribes into the following mRNA: AUG GGG CCC, which would translate into start-glycine-proline. As glycine is encoded by four codons (GGG, GGA, GGC, GGU), any change in the third position of that codon will have no effect. The same is true for the codon for proline. But what about changes in the other nucleotides in the sequence? They could have potentially dramatic effects. The effects depend on the outcome of the mutation. Obviously any change to the start codon will interrupt the start of translation. Turning the simple glycine into the nonpolar (and relatively large) tryptophan (UGG codon) could have dramatic effects on the function of the protein.
Once again, a mutation is the change in the DNA or RNA sequence. As discussed earlier, in multicellular organisms, mutations can be subdivided into germline mutations and so- matic mutations. Germline mutations occur in the DNA of sex cells, or gametes, and are
therefore potentially very serious. These mutations can be passed to the next generation. Somatic mutations, which occur in somatic, or body, cells, cannot be passed to the next generation (offspring). Mutations can be harmful, beneficial, or have no effect. If a mutation does not change the amino acid sequence in a protein, the mutation will have no effect. In fact, the overwhelming majority of mutations have no significant effect, since DNA repair mechanisms are able to mend most of the changes before they become permanent. Fur- thermore, many organisms have mechanisms for eliminating otherwise permanently mutated somatic cells.
A gene pool is the complete set of unique alleles in a species or population. Mutations create variation in the gene pool. Populations with a large gene pool are said to be genet- ically diverse and very robust. They are able to survive intense times of natural selection against certain phenotypes. During these times of selection, individuals with less favorable phenotypes resulting from deleterious alleles (due to mutations) may be selected against and removed from the population. Concurrently, the more favorable mutations that cause ben- eficial or advantageous phenotypes tend to accumulate in that population, resulting, over time, in evolution. We will discuss evolution and the genetic effects on evolution in much more detail in a later chapter.
Mutations can result in errors in protein sequence, creating partially or completely non- functional proteins. These can obviously result in harm to the cell and organism. As dis- cussed in the previous lesson, to function correctly and maintain homeostasis, each cell depends on thousands of proteins to all work together to perform the functions of the cell. When a mutation alters a protein that plays a critical role in the cell, the tissue, organ, or organ system may not function properly, resulting in a medical condition. A condition caused by mutations in one or more genes is called a genetic disorder, which will be dis- cussed in the next chapter. However, only a small percentage of mutations cause genetic disorders; most have no impact on health. If a mutation does not change the protein se- quence or structure, resulting in the same function, it will have no effect on the cell. Often, these mutations are repaired by the DNA repair system of the cell. Each cell has a number of pathways through which enzymes recognize and repair mistakes in DNA (Figure 8.20). Because DNA can be damaged or mutated in many ways, the process of DNA repair is an important way in which the cell protects itself to maintain proper function.
A mutation present in a germ cell can be passed to the next generation. If the zygote contains the mutation, every cell in the resulting organism will have that mutation. If the mutation results in a disease phenotype, the mutation causes what is called a hereditary disease. These will be discussed in the next chapter. On the other hand, a mutation that is present in a somatic cell of an organism will be present (by DNA replication and mitosis) in all descendants of that cell. If the mutation is present in a gene that is not used in that cell type, the mutation may have no effect. On the other hand, the mutation may lead to
www.ck12.org 386
Figure 8.20: DNA repair. Shown is a model of DNA ligase repairing chromosomal damage. DNA ligase is an enzyme that joins broken nucleotides together by catalyzing the formation of a bond between the phosphate group and deoxyribose sugar of adjacent nucleotides in the DNA backbone. (22)
a serious medical condition such as cancer. Mutations and cancer will be discussed in the next lesson.
A very small percentage of all mutations actually have a positive effect. These mutations lead to new versions of proteins that help an organism and its future generations better adapt to changes in their environment. The genetic diversity that results from mutations is essential for evolution to occur. Without genetic diversity, each individual of a species would be the same, and no one particular individual would have an advantage over another. Adaptation and evolution would not be possible. Beneficial mutations lead to the survival of the individual best fit to the current environment, which results in evolution. This will be discussed in the evolution chapter.
During the discussion of the cell cycle, cancer was described as developing due to unregulated cell division. That is, cancer is a disease characterized by a population of cells that grow and divide without respect to normal limits. These cancerous cells invade and destroy adjacent
tissues, and they may spread throughout the body.
Nearly all cancers are caused by mutations in the DNA of the abnormal cells. These mu- tations may be due to the effects of carcinogens, cancer causing agents such as tobacco smoke, radiation, chemicals, or infectious agents. These carcinogens may act as an environ- mental “trigger,” stimulating the onset of cancer in certain individuals and not others. Do all people who smoke get cancer? No. Complex interactions between carcinogens and an individual’s genome may explain why only some people develop cancer after exposure to an environmental trigger and others do not. Do all cancers need an environmental trigger to develop? No. Cancer causing mutations may also result from errors incorporated into the DNA during replication, or they may be inherited. Inherited mutations are present in all cells of the organism.
Mutations found in the DNA of cancer cells typically affect two general classes of genes: onco- genes and tumor suppressor genes. In “normal,” non-cancerous cells, the products of proto- oncogenes promote cell growth and mitosis prior to cell division; thus, proto-oncogenes en- code proteins needed for normal cellular functions. Mutations in proto-oncogenes can modify their expression and the function of the gene product, increasing the amount of activity of the product protein. When this happens, they become oncogenes; thus, the cells have a higher chance of dividing excessively and uncontrollably. Cancer-promoting oncogenes are often activated in cancer cells, giving those cells abnormal properties. The products of these genes result in uncontrolled cell growth and division, protection against programmed cell death, loss of respect for normal tissue boundaries, and the ability to become established in diverse tissue environments. Proto-oncogenes cannot be removed from the genome, as they are critical for growth, repair and homeostasis. It is only when they become mutated that the signals for growth become excessive.
In “normal” cells, the products of tumor suppressor genes temporarily discourage cell growth and division to allow cells to finish routine functions, especially DNA repair. Tumor suppressors are generally transcription factors, activated by cellular stress or DNA damage. The function of such genes is to stop the cell cycle in order to carry out DNA repair, pre- venting mutations from being passed on to daughter cells. However, if the tumor suppressor genes are inactivated, DNA repair cannot occur. Tumor suppressor genes can be inactivated by a mutation that either affects the gene directly or that affects the pathway that activates the gene. The consequence of the lack of DNA repair is that DNA damage accumulates, is not repaired, and inevitably leads to cancer.
www.ck12.org 388
Typically, a series of several mutations in these genes that activate oncogenes and inactivate tumor suppressor genes is required to transform a normal cell into a cancer cell (Figure 8.21). Cells have developed a number of control mechanisms to overcome mutations in proto-oncogenes. Therefore, a cell needs multiple mutations to transform into a cancerous cell. A mutation in one proto-oncogene would not cause cancer, as the effects of the mutation would be masked by the normal control of mitosis and the actions of tumor suppressor genes. Similarly, a mutation in one tumor suppressor gene would not cause cancer either, due to the presence of many ”backup” genes that duplicate its functions. It is only when enough proto-oncogenes have mutated into oncogenes and enough tumor suppressor genes have been deactivated that the cancerous transformation can begin. Signals for cell growth overwhelm the signals for growth regulation, and the cell quickly spirals out of control. Often, because many of these genes regulate the processes that prevent most damage to the genes themselves, DNA damage accumulates as one ages.
Usually, oncogenes are dominant alleles, as they contain gain-of-function mutations. Mean- while, mutated tumor suppressors are generally recessive alleles, as they contain loss-of- function mutations. A proto-oncogene needs only a mutation in one copy of the gene to generate an oncogene; a tumor suppressor gene needs a mutation in both copies of the gene to render both products defective. There are instances when, however, one mutated allele of a tumor suppressor gene can render the other copy non-functional. These instances result in what is known as a dominant negative effect.
Mutations may be due to environmental factors (mutagens) or may occur sponta- neously.
Typical mutagens include chemicals, such as those inhaled by smoking, and radiation, like X-rays, ultraviolet light, and nuclear radiation.
Germline mutations can be passed on to descendants; somatic mutations cannot be transmitted to the next generation.
Chromosomal alterations are large changes in the chromosome structure. There are 5 types of chromosomal alterations: deletions, duplications, insertions, inversions, and translocations.
Point mutations occur at a single site within the DNA; examples of these include silent mutations, missense mutations, and nonsense mutations.
A deletion or insertion in the DNA can alter the reading frame.
Loss-of-function and gain-of-function mutations may result in altered function of the gene product or protein.
Beneficial mutations may accumulate in a population, resulting, over time, in evolution.
Harmful mutations can result in errors in protein sequence, creating partially or com-
www.ck12.org 390
pletely non-functional proteins.
Nearly all cancers are caused by mutations in the DNA of the abnormal cells.
In non-cancerous cells, proto-oncogenes promote cell growth and mitosis prior to cell division; thus, proto-oncogenes encode proteins needed for normal cellular functions.
In non-cancerous cells, tumor suppressor genes temporarily discourage cell growth and division to allow cells to finish routine functions, especially DNA repair.
Mutations in proto-oncogenes and tumor suppressor genes may lead to cancer.
Usually mutations in multiple genes are necessary to develop cancer.
Define mutation.
What are some common causes of mutations?
List some common types of mutations.
Describe some common chromosomal alterations.
Discuss potential consequences of point mutations, deletions and insertions.
List and describe three common types of point mutations.
What are effect-on-function mutations?
What is a germline mutation? A somatic mutation?
Explain why some mutations are harmful and some beneficial.
Campbell, N.A. and Reece, J.B. Biology, Seventh Edition, Benjamin Cummings, San Francisco, CA, 2005.
Biggs, A., Hagins, W.C., Kapicka, C., Lundgren, L., Rillero, P., Tallman, K.G., and Zike, D., Biology: The Dynamics of Life, California Edition, Glencoe Science, Colum- bus, Oh, 2005.
Nowicki S., Biology, McDougal Littell, Evanston, IL, 2008.
Kimball’s Biology Pages:
How Cancer Starts:
Mutations and evolution:
allele An alternative form of a gene.
beneficial mutation A mutation that leads to a new version of a protein that helps an organism and its future generations better adapt to changes in their environment.
cancer A disease characterized by a population of cells that grow and divide without respect to normal limits.
carcinogen Cancer causing agent, such as tobacco smoke, radiation, chemicals, or infec- tious agents.
chromosomal alterations Large changes in chromosome structure.
deamination A mutation due to the spontaneous deamination of 5-methycytosine.
deletion Removal of a large chromosomal region, leading to loss of the genes within that region; also the removal of one or more nucleotides from DNA.
depurination A mutation due to the loss of a purine base (A or G).
DNA ligase An enzyme that joins broken nucleotides together by catalyzing the formation of a bond between the phosphate group and deoxyribose sugar of adjacent nucleotides in the DNA backbone.
dominant negative mutation Mutation that results in an altered gene product that acts in a dominant manner to the wild-type gene product.
duplication Leads to multiple copies of a chromosomal region, increasing the number of the genes located within that region; also know as amplification.
frameshift mutation Mutations which alter the mRNA reading frame.
gain-of-function mutation Mutation that results in the gene product or protein having a new and abnormal function.
gene pool The complete set of unique alleles in a species or population.
genetic disorder A condition caused by mutations in one or more genes.
germline mutation Mutation in the DNA within a gamete; can be passed on to descen- dents.
www.ck12.org 392
insertion Chromosomal alteration involving the addition of material from one chromosome to a nonhomologous chromosome; also a mutation which adds one or more nucleotides into the DNA.
inversion Chromosomal alteration reversing the orientation of a chromosomal segment.
loss-of-function mutation Mutation that results in a gene product or protein having less or no function.
missense mutations Point mutation which codes for a different amino acid.
mutagen An environmental factor which causes a mutation; includes certain chemicals and radiation.
mutation A change in the DNA or RNA sequence.
nonsense mutation Point mutation which codes for a premature stop codon.
oncogene Cancer promoting gene; the products of these genes result in uncontrolled cell growth and division, protection against programmed cell death, loss of respect for normal tissue boundaries, and the ability to become established in diverse tissue envi- ronments.
point mutations Exchange one nucleotide for another; known as base substitution muta- tions.
proto-oncogenes Genes whose products promote cell growth and mitosis prior to cell division.
silent mutations Point mutation which codes for the same amino acid.
somatic mutation A mutation in a body cell, not in a gamete; cannot be transmitted to the next generation.
splice site mutation Mutation in the coding region of a gene that alters splicing of the mRNA.
tautomerism A mutation due to the changing of a base by the repositioning of a hydrogen atom.
transition A purine to purine, or a pyrimidine to pyrimidine change.
translocation Chromosomal alterations involving the interchange of genetic material be- tween nonhomologous chromosomes.
transversion A purine is replaced by a pyrimidine, or a pyrimidine is replaced by a purine.
tumor suppressor gene Gene whose product temporarily discourage cell growth and di- vision to allow cells to finish routine functions, especially DNA repair.
Now that we have discussed DNA, protein synthesis and mutations, can you think of a mechanism that allows different cell types to have different proteins?
What about during development? Why does a developing embryo need different pro- teins at different times of development?
We have discussed oncogenes and tumor suppressor genes. Can you think of a specific cellular mechanism in which defects in these genes lead to cancer?
Describe general mechanisms of gene expression.
Differentiate between a cis-regulatory element and a trans-acting factor.
Define a transcription factor.
Define an operon.
Describe how the lac operon regulates transcription.
Describe the role of the TATA box.
Express the importance of gene regulation during development.
Describe the role of homeobox genes and gap genes.
Discuss gene regulation in terms of the development of cancer.
Each of your cells has about 22,000 genes. In fact, all of your cells have the same genes. So do all of your cells make the same proteins? Do all 22,000 genes get turned into proteins in every cell? Of course not. If they did, then all your cells would do the same thing. You have cells with different functions because you have cells with different proteins. And your cells
www.ck12.org 394
have different proteins because they “use” different genes. The regulation of gene expression, or gene regulation, includes the mechanism to turn genes “on” and transcribe the gene into RNA. Any aspect of a gene’s expression may be regulated, from the onset of transcription to the post-translational modification of a protein. It is this regulation that determines when and how much of a protein to make, giving a cell its specific structure and function.
Any step of gene expression may be modulated, from the DNA-RNA transcription step to post-translational modification of a protein. Following is a list of stages where gene expression is regulated:
Chemical and structural modification of DNA or chromatin
Transcription
Translation
Post-transcriptional modification
RNA transport
mRNA degradation
Post-translational modifications
In this lesson, we will focus on regulation at the level of transcription. We have previously discussed that during transcription RNA polymerase reads the DNA template to make a complementary strand of RNA. The genes to which RNA polymerase binds is a highly regulated process. When RNA polymerase binds to a gene, it binds to the promoter, a segment of DNA that allows a gene to be transcribed. The promoter helps RNA polymerase find the start of a gene.
Gene regulation at the level of transcription controls when transcription occurs as well as how much RNA is created. This regulation is controlled by cis-regulatory elements and trans-acting factors. A cis-regulatory element is a region of DNA which regulates the expression of a gene or multiple genes located on that same strand of DNA. These cis- regulatory elements are often the binding sites of one or more trans-acting factors, usually a regulatory protein which interacts with RNA polymerase. A cis-regulatory element may be located in a gene’s promoter region, in an intron, or in the 3’ region.
A regulatory protein, or a transcription factor, is a protein involved in regulating gene expression. It is usually bound to a cis-regulatory element. Regulatory proteins often must be bound to a cis-regulatory element to switch a gene on (activator), or to turn a gene off (repressor).
Transcription of a gene by RNA polymerase can be regulated by at least five mechanisms:
Specificity factors (proteins) alter the specificity of RNA polymerase for a promoter
or set of promoters, making it more or less likely to bind to the promoter and begin transcription.
Repressors (proteins) bind to non-coding sequences on the DNA that are close to or overlap the promoter region, impeding RNA polymerase’s progress along the strand.
Basal factors, transcription factors that help position RNA polymerase at the start of a gene.
Enhancers are sites on the DNA strand that are bound by activators in order to loop the DNA, bringing a specific promoter to the initiation complex. An initiation complex is composed of RNA polymerase and trans-acting factors.
Activators (proteins) that enhance the interaction between RNA polymerase and a particular promoter.
As the organism grows more sophisticated, gene regulation becomes more complex, though prokaryotic organisms possess some highly regulated systems. Some human genes are con- trolled by many activators and repressors working together. Obviously, a mutation in a cis-regulatory region, such as the promoter, can greatly affect the proper expression of a gene. It may keep the gene permanently off, such that no protein can be made, or it can keep the gene permanently on, such that the corresponding protein is constantly made. Both of these can have detremental effects on the cell.
In prokaryotes, a combination of activators and repressors determines whether a gene is transcribed. As you know, prokaryotic organisms are fairly simple organisms with much less DNA. Prokaryotic genes are arranged in operons, a region of DNA with a promoter, an operator (defined below), and one or more genes that encode proteins needed to perform a certain task. To maintain homeostasis (and survive), the organism must quickly adapt changing environmental conditions. The regulation of transcription plays a key role in this process.
For a bacteria, many aspects of gene regulation are due to the presence or absence of certain nutrients. In prokaryotes, repressors bind to regions called operators that are generally lo- cated immediately downstream from the promoter. Activators bind to the upstream portion of the promoter.
The lac operon (Figure 8.22) is an operon required for the transport and metabolism of lactose in E. coli. The lac operon is regulated by the availability of lactose. The lac operon consists of a promoter, an operator, three adjacent structural genes which code for enzymes and a terminator. The three genes are: lacZ, lacY, and lacA. All three genes are controlled by the same regulatory elements.
www.ck12.org 396

In bacteria, the lac repressor protein blocks the synthesis of enzymes that digest lactose when there is no lactose present (Figure 8.23). When lactose is present, it binds to the repressor, causing it to detach from the DNA strand.
Specific control of the lac operon depends on the availability of lactose. The enzymes needed to metabolize lactose are not produced when lactose is not present. When lactose is available, and therefore needs to be metabolized, the operon is turned on, RNA polymerase binds to the promoter, and the three genes are transcribed into a single mRNA molecule. However, if lactose is not present (and therefore does not need to be metabolized), the operon is turned off by the lac repressor protein (Figure 8.23).
The lacI gene, which encodes the lac repressor, lies near the lac operon and is always expressed (constitutive). Therefore, the lac repressor protein is always present in the bacteria. In the absence of lactose, the lac repressor protein will bind to the operator, just past the promoter in the lac operon. The repressor blocks the binding of RNA polymerase to the promoter, keeping the operon turned off (Figure 8.23).
When lactose is available, a lactose metabolite called allolactose binds to the repressor. This interaction causes a conformational change in the repressor shape and the repressor falls off the operator, allowing RNA polymerase to bind to the promoter and initiate transcription (Figure 8.23).
As previously discussed, all your cells have the same DNA (and therefore the same genes), yet they have different proteins because they express different genes. In eukaryotic cells, the start of transcription is one of the most complex aspects of gene regulation. Transcriptional regulation involves the formation of an initiation complex involving interactions between a number of transcription factors, cis-regulatory elements, and enhancers, distant regions of DNA that can loop back to interact with a gene’s promoter. These regulatory elements occur in unique combinations within a given cell type, resulting in only necessary genes being transcribed in certain cells. Transcription factors bind to a DNA strand, allowing RNA polymerase to bind and start transcription.
Each gene has unique cis-regulatory sequences, only allowing specific transcription factors to bind. However, there are common regulatory sequences found in most genes. The TATA
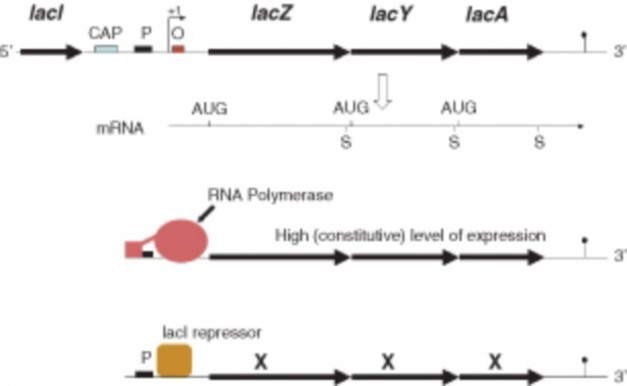
Figure 8.23: Regulation of the lac operon. When lactose is present, RNA polymerase (red) binds to the promoter (P) and the three genes are expressed, producing a single mRNA for the three genes. When lactose is unavailable, the lac repressor (yellow) binds to the operator
and inhibits the binding of RNA polymerase to the promoter. The three genes are not expressed. For an animation of the Lac Operon, see http://vcell.ndsu.edu/animations/ lacOperon/first.htm. (15)
www.ck12.org 398
box is a cis-regulatory element found in the promoter of most eukaryotic genes. It has the DNA sequence 5’-TATAAA-3’ or a slight variant, and has been highly conserved throughout evolution. When the appropriate cellular signals are present, RNA polymerase binds to the TATA box, completing the initiation complex. A number of transcription factors first bind to the TATA box while other transcription factors bind to the previously attached factors, forming a multi-protein complex. It is only when all the appropriate factors are bound that RNA polymerase will recognize the complex and bind to the DNA, initiating transcription.
One of the more complex eukaryotic gene regulation processes is during development. What genes must be turned on during development so that tissues and organs form from simple cells?
What makes the heart form during development? What makes the skin form? What makes a structure become an arm instead of a leg? These processes occur during development because of a highly specific pattern of gene expression. This intensely regulated pattern of gene expression turns genes on in the right cell at the right time, such that the resulting proteins can perform their necessary functions to ensure proper development. Transcription factors play an extremely important role during development. Many of these proteins can be considered master regulatory proteins, in the sense that they either activate or deactivate the transcription of other genes and, in turn, these secondary gene products can regulate the expression of still other genes in a regulatory cascade. Homeobox and gap genes are important transcription factors during development.
Homeobox genes contain a highly conserved DNA sequence known as a homeobox and are involved in the regulation of genes important to development. A homeobox is about 180 base pairs long; it encodes a 60 amino acid domain within the protein (known as the home- odomain), which can bind DNA. Proteins with a homeodomain are therefore transcription factors. These factors typically switch on series of other genes, for instance, the genes needed to encode the proteins to make a leg.
A particular subgroup of homeobox genes are the Hox genes. Protein products of Hox genes function in patterning the body, providing the placement of certain body parts during development. In other words, Hox genes determine where limbs and other body segments will grow in a developing fetus or larva. Mutations in any one of these genes can lead to the growth of extra, typically non-functional body parts in invertebrates. The Antennapedia mutation in Drosophila results in a leg growing from the head in place of an antenna. A mutation in a vertebrate Hox genes usually results in miscarriage.
A gap gene controls the shape of a developing zygote early in its development. The products of these genes produce gaps in a rather uniform arrangement of cells (Figure 8.24). One example of this is the Kruppel gene, which regulates the activity of a number of other genes. Gap genes encode transcription factors, and the Kruppel gene is a zinc-finger protein. A zinc finger is a DNA binding region within the protein. A zinc finger consists of two antiparallel sheets and an helix with a zinc ion, which is important for the stability of this region. Gap genes control the expression of other genes within specific regions of cells in the developing organism. This allows specific genes to be expressed in certain cells at the appropriate stage of development.
Figure 8.24: Gap gene expression. Shown is the expression pattern of four gap genes, Kruppel, Giant, Knirps, and Tailless, in a developing Drosophilia embryo. Note how the expression of these genes creates an unique pattern resulting in gaps in what was a rather uniform arrangement of cells. (25)
As discussed in the last lesson, carcinogenesis depends on both the activation of oncogenes and deactivation of tumor suppressor genes. At least two separate mutations are necessary to develop cancer. For example, a mutation in a proto-oncogene would not necessarily lead to cancer, as normally functioning tumor suppressor genes would counteract the effects of the oncogene. It is the second mutation in the tumor suppressor gene that could lead to uncontrolled cell growth and possibly cancer. Both oncogenes and tumor suppressor genes play an important role in gene regulation and cell proliferation (Figure 8.25).
www.ck12.org 400
The products of proto-oncogenes are required for normal growth, repair and homeostasis. However, when these genes are mutated, they turn into oncogenes and play a role in the development of cancer. Proto-oncogenes may be growth factors, transcription factors, or other proteins involved in regulation. A very common oncogene, ras, is normally a regulatory GTPase that switches a signal transduction chain on and off. Ras and Ras-related proteins are products of oncogenes found in 20% to 30% of human tumors.
Ras is a G protein, a regulatory GTP hydrolase that cycles between an activated and inactivated form. When a growth factor binds to its receptor on the outside of the cell, a signal is relayed to RAS. As a G protein, Ras is activated when GTP is bound to it. The active Ras then passes the signal to a series of protein kinases, regulatory proteins that eventually activate transcription factors to alter gene expression and produce proteins that stimulate the cell cycle (Figure 8.25). Many of the genes and proteins involved in signal transduction pathways are interconnected to ras. Any mutation that makes ras more active or otherwise interrupts the normal signal transduction pathways (Figure 8.25) may result in excessive cell division and cancer.
An example of a tumor suppressor gene is p53, which encodes a 53,000 dalton protein, The p53 gene is activated by DNA damage. DNA may be damaged by ultraviolet light, and any damaged DNA may be harmful to the cell. Mutations causing problems with any of the components of Figure 8.25 may lead to the development of cancer. So that damaged DNA is not replicated, the cell cycle must be temporarily stopped so that the DNA can be repaired. The p53 tumor suppressor gene encodes a transcription factor that regulates the synthesis of cell cycle inhibiting proteins (Figure 8.25). p53 often activates a gene named p21, whose protein product temporarily stops the cell cycle. If the DNA can not be repaired, p53 activates other genes that lead to cell death, or apoptosis. This prevents the cell from passing on damaged DNA. If the p53 tumor suppressor gene is defective, as by mutation, DNA damage in the cell may accumulate and the cell may survive to replicate the damaged DNA. The damaged DNA would then be passed to other cells through many cell divisions, and cancer could develop.
A cis-regulatory element is a region of DNA which regulates the expression of a gene or multiple genes located on that same strand of DNA.
The cis-regulatory elements are often binding sites for one or more trans-acting factors, usually a regulatory protein which interacts with RNA polymerase.
Repressor proteins bind to non-coding sequences on the DNA that are close to or
www.ck12.org 402
overlap the promoter region, impeding RNA polymerase’s progress along the strand.
Enhancers are sites on the DNA strand that are bound by activators.
Prokaryotic genes are arranged in operons, regions of DNA with a promoter, an oper- ator, and one or more genes that encode proteins needed to perform a certain task.
The regulation of the lac operon is a key example of prokaryotic gene regulation. When lactose is present, RNA polymerase binds to the promoter and the operon is turned on; when lactose is unavailable, the lac repressor binds to the operator and the operon is turned off.
Homeobox genes are involved in the regulation of genes important to development. They encode transcription factors.
Gap genes control the shape of a developing zygote early in its development.
At least two separate mutations are necessary to develop cancer. These mutations may occur in proto-oncogenes and/or tumor suppressor genes.
What is meant by gene expression, and why is this an important cellular mechanism?
How do cis-regulatory elements and a trans-acting factors work together?
Define a transcription factor.
Define an operon. Give an example.
Describe how the lac operon regulates transcription.
Describe the role of the TATA box.
Why is gene regulation an important aspect of development?
What is a homeobox gene? A gap gene? Why are these genes important?
Why does altered gene regulation have a potential role in the development of cancer?
Campbell, N.A. and Reece, J.B. Biology, Seventh Edition, Benjamin Cummings, San Francisco, CA, 2005.
Biggs, A., Hagins, W.C., Kapicka, C., Lundgren, L., Rillero, P., Tallman, K.G., and Zike, D., Biology: The Dynamics of Life, California Edition, Glencoe Science, Colum- bus, Oh, 2005.
Nowicki S., Biology, McDougal Littell, Evanston, IL, 2008.
activator Protein that enhances the interaction between RNA polymerase and a particular promoter.
basal factor Transcription factor that helps position RNA polymerase at the start of a gene.
cis-regulatory element A region of DNA which regulates the expression of a gene or multiple genes located on that same strand of DNA.
enhancer Site on the DNA strand that can be bound by activator(s) in order to loop the DNA, bringing a specific promoter to the initiation complex.
G protein A regulatory GTP hydrolase that cycles between an activated and inactivated form; activated when GTP is bound to it.
gap gene Gene that controls the shape of a developing zygote early in its development; encodes transcription factors.
homeobox A 180 base pair long highly conserved segment of DNA; encodes a 60 amino acid domain within the protein (known as the homeodomain), which can bind DNA.
homeobox genes Genes that contain a highly conserved DNA sequence known as a home- obox and are involved in the regulation of genes important to development; encodes transcription factors.
hox genes Genes that function in patterning the body, providing the placement of certain body parts during development.
initiation complex Complex needed to start transcription in eukaryotes; composed of RNA polymerase and trans-acting factors.
lac operon An operon required for the transport and metabolism of lactose in E. coli.
operator A region of prokaryotic DNA where a repressors binds.
operon A region of prokaryotic DNA with a promoter, an operator, and one or more genes that encode proteins needed to perform a certain task.
promoter A segment of DNA that allows a gene to be transcribed; helps RNA polymerase find the start of a gene.
repressor Protein that binds to non-coding sequences on the DNA that are close to or overlap the promoter region, impeding RNA polymerase’s progress along the strand.
www.ck12.org 404
RNA polymerase The enzyme that transcribes the DNA to make RNA.
specificity factor Protein that alters the specificity of RNA polymerase for a promoter or set of promoters, making it more or less likely to bind to the promoter and begin transcription.
TATA box A cis-regulatory element found in the promoter of most eukaryotic genes; when the appropriate cellular signals are present, RNA polymerase binds to the TATA box, completing the initiation complex.
transcription factor A protein involved in regulating gene expression; usually bound to a cis-regulatory element; also known as a regulatory protein.
zinc finger A DNA binding region within certain proteins encoded by a gap gene; consists of two antiparallel sheets and an helix with a zinc ion, which is important for the stability of this region.
The next chapter is Human Genetics. Discuss why an understanding of human genetics is an important medical issue for our society.
We have extensively discussed mutations and cancer. There are many other phenotypes due to mutations in the human genome. Why is understanding mutations in humans important?
What do you think the Human Genome Project is? What could some implications of The Human Genome Project be?
http://en.wikipedia.org/wiki/Image:Griffith_experiment.svg. GNU-FDL.
http://en.wikipedia.org/wiki/Image:Pre-mRNA_to_mRNA.png. GNU-FDL.
http://commons.wikimedia.org/wiki/Image:Transcription.png. Public Domain.
http://en.wikipedia.org/wiki/Image:Schema_ARNt_448_658.png. GNU-GPL.
Summary of translation.. Public Domain.
NIH. http://en.wikipedia.org/wiki/Image: Cancer_requires_multiple_mutations_from_NIH.png. Public Domain.
http://www.genome.gov//Pages/Hyperion/DIR/VIP/Glossary/Illustration/ mutation.cfm. Public Domain.
http://en.wikipedia.org/wiki/Image:Tevenphage.png. CC-BY-SA 2.5.
http://en.wikipedia.org/wiki/Image:Lac_operon1.png. Public Domain.
http://en.wikipedia.org/wiki/Image:
NA-comparedto-DNA_thymineAndUracilCorrected.png. CC-BY-SA.
CK-12 Foundation. http://en.wikipedia.org/wiki/Image:HersheyChaseEx.png. CC-BY-SA 2.5.
CK-12 Foundation. http://en.wikipedia.org/wiki/Image:Guanine_chemical_structure.png http://en.wikipedia.org/wiki/Image:Cytosine_chemical_structure.png http://en.wikipedia.org/wiki/Image:Thymine.png. GNU-FDL.
NIH. http://en.wikipedia.org/wiki/Image:JamesDWatson.jpg. CC-BY 2.5,Public Domain.
http://en.wikipedia.org/wiki/Image:3d_tRNA.png. CC-BY-SA 3.0.
http://commons.wikimedia.org/wiki/File:DNA_replication_en.svg. Public Domain.
http://en.wikipedia.org/wiki/Image:Signal_transduction_v1.png. GNU-FDL.
The four stages of protein folding.. Public Domain.
http://en.wikipedia.org/wiki/Image:DNA_Overview.png. GNU-FDL.
http://en.wikipedia.org/wiki/Image:RNA-codons.png. Public Domain.
http://en.wikipedia.org/wiki/Image:DNA_Repair.jpg. Public Domain.
http://en.wikipedia.org/wiki/Genetic_code. CC-BY-SA.
http://en.wikipedia.org/wiki/Image:DNA_chemical_structure.svg. GNU-FDL.
http://commons.wikimedia.org/wiki/Image:Gap_ene_expression.png. Public Domain.
www.ck12.org 406
What is a genetic disease?
What is the human genome?
Discuss the importance of characterizing the human genome.
Define autosome and sex-chromosome.
Discuss the importance of SNPs.
What is a karyotype?
Define sex-linked and X-inactivation.
As has been previously discussed, genetics is the branch of biology that focuses on heredity. The basics of heredity are similar for all organisms that reproduce sexually: the offspring receive one set of genetic material from one parent and the other set from the other parent. But are there aspects of genetics that are specific for us? Let’s find out.
A genetic disease is a phenotype due to a mutation in a gene or chromosome. Many of these mutations are present at conception and are therefore in every cell of the body. Mutant alleles may be inherited from one or both parents, resulting in a dominant or recessive hereditary disease. Currently, there are over 4,000 known genetic disorders, with many more phenotypes yet to be identified. Theoretically, every human gene, when disrupted due to a mutation, could result in at least one disease-type phenotype. Genetic diseases are typically diagnosed and treated by a geneticist, a medical doctor specializing in these disorders, many of which are extremely rare and difficult to diagnose. Individuals and families with genetic diseases, or
suspected genetic diseases, are often counseled by genetic counselors, individuals trained in human genetics and counseling. To understand human genetic diseases, you first need to understand human chromosomes and genes.
What makes each one of us unique? You could argue that the environment plays a role, and it does to some extent. But most would agree that your parents have something to do with your uniqueness. In fact, it is our genes that make each one of us unique – or at least genetically unique. We all have the genes that make us human: the genes for skin and bones, eyes and ears, fingers and toes, and so on. However, we all have different skin colors, different bone sizes, different eye colors and different ear shapes. In fact, even though we have the same genes, the products of these genes work a little differently in most of us. And that is what makes us unique.
The human genome is the genome - all the DNA - of Homo sapiens. Humans have about 3 billion bases of information, divided into roughly 20,000 genes, which are spread among non-coding sequences and distributed among 24 distinct chromosomes (22 autosomes plus the X and Y sex chromosomes) (Figure 9.1). The genome is all of the hereditary infor- mation encoded in the DNA, including the genes and non-coding sequences. The Human Genome Project (See the Biotechnology chapter) has produced a reference sequence of the human genome. The human genome consists of protein-coding exons, associated introns and regulatory sequences, genes that encode other RNA molecules, and “junk” DNA, regions in which no function as yet been identified.
The human genome consists of 24 distinct chromosomes: 22 autosomal chromosomes plus the sex-determining X and Y chromosomes. A chromosome is a threadlike molecule of genes and other DNA located in the nucleus of a cell. Different organisms have different numbers of chromosomes. Human somatic cells have 23 chromosome pairs for a total of 46 chromosomes: two copies of the 22 autosomes (one from each parent), plus an X chromosome from the mother and either an X or Y chromosome from the father (Figure 9.2).
There are an estimated 20,000 human protein-coding genes, but many more proteins. Most human genes have multiple exons separated by much larger introns. Regulatory sequences controlling gene expression are associated with exon sequences. The introns are usually excised (removed) during post-transcriptional modification of the mRNA. Human cells make significant use of alternative splicing (see the Molecular Genetics chapter) to produce a number of different proteins from a single gene. So even though the human genome is surprisingly similar in size to the genomes of simpler organisms, the human proteome is thought to be much larger. A proteome is the complete set of proteins expressed by a
www.ck12.org 408
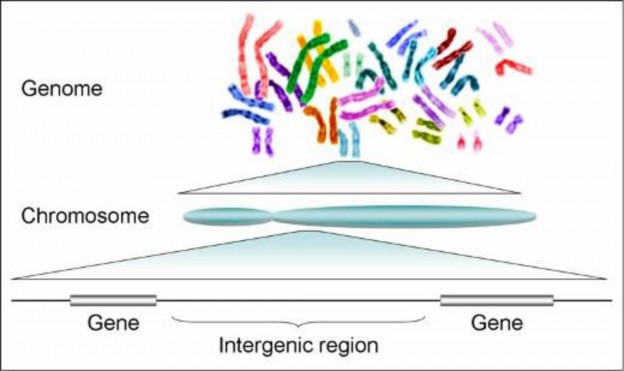
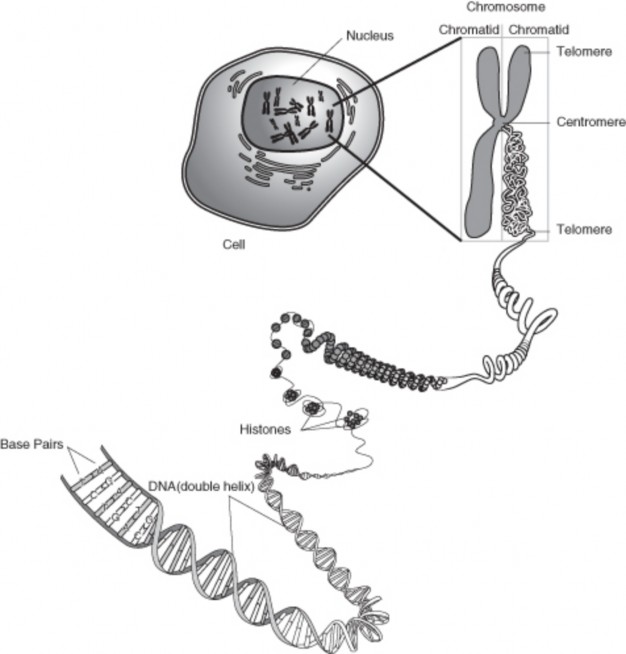
www.ck12.org 410
genome, cell, tissue, or organism.
As stated above, our roughly 20,000 genes are located on 24 distinct chromosomes. Link- age refers to particular genetic loci, or alleles inherited together, suggesting that they are physically on the same chromosome, and located close together on that chromosome. Two or more loci that are on the same chromosome are physically connected and tend to segregate together during meiosis, unless a cross over event occurs between them. A crossing-over event during prophase I of meiosis is rare between loci that usually segregate together; these loci will usually be close together on the same chromosome. They are, therefore, said to be linked. Alleles for genes on different chromosomes are not linked; they sort independently (independent assortment) of each other during meiosis.
A gene is also said to be linked to a chromosome if it is physically located on that chromosome. For example, a gene (or loci) is said to be linked to the X-chromosome if it is physically located on the X-chromosome chromosome. The physical location of a gene is important when analyzing the inheritance patterns of phenotypes due to that gene. The inheritance patterns of phenotypes may be different if the gene is located on a sex chromosome or an autosome. This will be further discussed in the next lesson.
The frequency of recombination refers to the rate of crossing-over (recombination) events between two loci. This frequency can be used to estimate genetic distances between the two loci, and create a linkage map. In other words, the frequency can be used to estimate how close or how far apart the two loci are on the chromosome.
In the early 20th century, Thomas Hunt Morgan, working with the fruit fly Drosophila Melanogaster, demonstrated that the amount of crossing over between linked genes differs. This led to the idea that the frequency of crossover events would indicate the distance separating genes on a chromosome. Morgan’s student, Alfred Sturtevant, developed the first genetic map, also called a linkage map.
Sturtevant proposed that the greater the distance between linked genes, the greater the chance that non-sister chromatids would cross over in the region between the genes during meiosis. By determining the number of recombinants - offspring in which a cross-over event has occured - it is possible to determine the approximate distance between the genes. This distance is called a genetic map unit (m.u.), or a centimorgan, and is defined as the distance between genes for which one product of meiosis in 100 products is a recombinant. So, a recombinant frequency of 1% (1 out of 100) is equivalent to 1 m.u. Loci with a recombinant frequency of 10% would be separated by 10 m.u. The recombination frequency will be 50% when two genes are widely separated on the same chromosome or are located on
different chromosomes. This is the natural result of independent assortment. Linked genes have recombination frequencies less than 50%.
Determining recombination frequencies between genes located on the same chromosome al- lows a linkage map to be developed. Linkage mapping is critical for identifying the location of genes that cause genetic diseases.
As stated above, even though we essentially all have the same genes, the gene products work a little different in all of us, making us unique. That is, the variation within the human genome results in the uniqueness of our species. In fact, genetically speaking, we are all about 99.9% identical. However, it is this 0.1% variation that results in our physical noticeable differences, as well as traumatic events such as illnesses or congenital deformities. These differences can also be used for societies benefits, such as through forensic DNA analysis (discussed in the Biotechnology chapter). Most studies of this genetic variation focus on small differences, know as SNPs, or single nucleotide polymorphisms, which are substitutions in individual bases along a chromosome. For example, the single base change from the sequence GGATAACGTCA to GGAAAACGTCA would be a SNP. Although not occurring uniformly, in the human genome, it has been estimated that SNPs occur every 1 in 100 to 1 in 1000 bases.
DNA sequences that repeat a number of times are known as repetitive sequences or repet- itive elements. For example the sequence CACACACACACACA would be a dinucleotide (2 base) repeat, or the sequence GATCGATCGATCGATCGATC would be a tetranucleotide (4 base) repeat. The genomic loci and length of certain types of repetitive sequences are highly variable from person to person, which is the basis of DNA fingerprinting and DNA paternity testing technologies. Longer repetitive elements are also common in the human genome. Examples of repeat polymorphisms are described in Table 9.1
Table 9.1: Repeat Polymorphisms (bp = base pair)
![]()
Dinucleotide repeats of two bp sequences
![]()
Tetranucleotide repeats of four bp sequences
Microsatellite; Short Tandem Repeats (STRs)
short sequences of 100-200 bp, usually due to repeats of 1-6 bp sequences
Minisatellite short sequences of 6-10 bp repeats
VNTR: Variable Number of Tandem Re- peat
short nucleotide sequence ranging from 14 to 100 nucleotides long, organized into clus- ters of tandem repeats, usually repeated in the range of between 4 and 40 times per loci
![]()
www.ck12.org 412
There are 44 autosomes and 2 sex chromosomes in the human genome, for a total of 46 chromosomes (23 pairs). Sex chromosomes specify an organism’s genetic sex. Humans can have two different sex chromosomes, one called X and the other Y. Normal females possess two X chromosomes and normal males one X and one Y. An autosome is any chromosome other than a sex chromosome. Figure 3 9.3 shows a representation of the 24 different human chromosomes. Figure 9.4 shows a karyotype of the human genome. A karyotype depicts, usually in a photograph, the chromosomal complement of an individual, including the number of chromosomes and any large chromosomal abnormalities. Karyotypes use chromosomes from the metaphase stage of mitosis.
The 22 autosomes are numbered based on size, with the largest chromosome labeled chro- mosome 1. These 22 chromosomes occur in homologous pairs in a normal diploid cell, with one of each pair inherited from each parent. The sex of an individual is determined by the sex chromosome within the male gamete. Females are homologous, XX, for the sex chro- mosomes, whereas males are heterozygous, XY. As all individuals inherit an X chromosome from their mother (females can only produce gametes with an X chromosome), it is the sex chromosome that they inherit from their father that determines their sex.
Both autosomal-linked and sex-linked traits and disorders will be discussed later in this chapter.
Sex-linked genes are located on either the X or Y chromosome, though it more commonly refers to genes located on the X-chromosome. For that reason, the genetics of sex-linked (or X-linked) diseases, disorders due to mutations in genes on the X-chromosome, results in a phenotype usually only seen in males. This will be discussed in the next lesson.
In humans, the Y chromosome spans 58 million bases and contains about 78 to 86 genes, which code for only 23 distinct proteins, making the Y chromosome one of the smallest chromosomes. The X chromosome, on the other hand, spans more than 153 million bases and represents about 5% of the total DNA in women’s cells, 2.5% in men’s cells. The X chromosome contains about 2,000 genes, however few, if any, have anything to do with sex determination. The Y chromosome is the sex-determining chromosome in humans and most other mammals. In mammals, it contains the gene SRY (sex-determining region Y), which encodes the testes-determining factor and triggers testis development, thus determining sex. It is the presence or absence of the Y chromosome that determines sex.
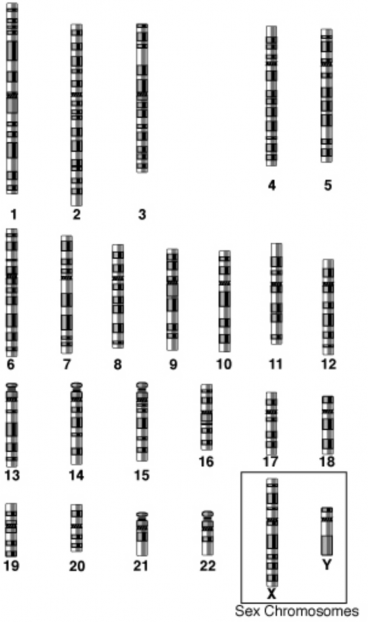
www.ck12.org 414
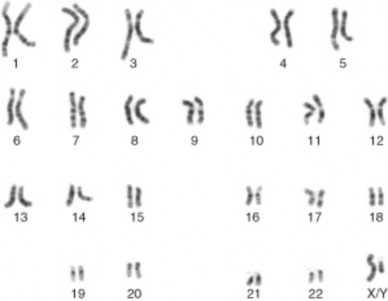
Figure 9.4: A karyotype of the human genome. Is this from a male or female? (12)
Early in embryonic development in females, one of the two X chromosomes is randomly inactivated in nearly all somatic cells. This process, called X-inactivation, ensures that females, like males, have only one functional copy of the X chromosome in each cell. X- inactivation creates a Barr body, named after their discover, Murray Barr. The Barr body chromosome is generally considered to be inactive, however there are a small number of genes that remain active and are expressed.
A genetic disease is a phenotype due to a mutation in a gene or chromosome.
Many of these mutations are present at conception, and are therefore in every cell of the body.
Mutant alleles may be inherited from one of both parents, resulting in a dominant or recessive hereditary disease.
Currently there are over 4,000 known genetic disorders, with many more phenotypes yet identified.
The genome refers to all the DNA of a particular species.
The human genome consists of 24 distinct chromosomes: 22 autosomal chromosomes, plus the sex-determining X and Y chromosomes.
Linkage refers to particular genetic loci or alleles inherited together, suggesting that they are physically on the same chromosome, and located close together on that chro-
mosome.
The variation within the human genome results in the uniqueness of our species.
There are 44 autosomes and 2 sex chromosomes in the human genome, for a total of 46 chromosomes.
Sex chromosomes specify an organism’s genetic sex. Humans have two different sex chromosomes, one called X and the other Y.
Sex-linked genes are located on either the X or Y chromosome, though it more com- monly refers to genes located on the X-chromosome.
Early in embryonic development in females, one of the two X chromosomes is randomly inactivated in nearly all somatic cells. This process is called X-inactivation.
What is a genetic disease?
Discuss the main difference between autosomal and sex-linked.
Why is variation within the human genome important?
Why is it more common for males to have X-linked disorders?
Describe how a mutation can lead to a genetic disease.
Discuss how a new mutation can become a new dominant allele.
How are autosomal traits usually inherited? Give examples of traits.
How are genetic diseases usually inherited? Are there exceptions? Research examples.
The National Human Genome research Institute:
The Dolan DNA Learning Center:
DNA Interactive:
A Science Odyssey: DNA Workshop:
Kimball’s Biology Pages:
autosome Any chromosome other than a sex chromosome.
Barr body The inactive X-chromosome in females. www.ck12.org 416
chromosome A threadlike molecule of genes and other DNA wound around histone pro- teins; located in the nucleus of a cell.
genetic counselor An individual trained in human genetics and counseling. genetic disease A phenotype due to a mutation in a gene or chromosome. geneticist A medical doctor specializing in genetic disorders.
genetics The branch of biology that focuses on heredity.
genome All of the hereditary information encoded in the DNA, including the genes and non-coding sequences.
karyotype Depicts, usually in a photograph, the chromosomal complement of an individ- ual, including the number of chromosomes and any large chromosomal abnormalities.
linkage Refers to particular genetic loci or alleles inherited together, suggesting that they are physically on the same chromosome, and located close together on that chromo- some.
microsatellite Short sequences of 100-200 bp, usually due to repeats of 1-6 bp sequences; also known as a STR (Short Tandem Repeat) polymorphism.
minisatellite Short sequence polymorphisms of 6-10 bp repeats.
proteome The complete set of proteins expressed by a genome, cell, tissue, or organism.
repetitive sequences DNA sequences that repeat a number of times; also known as repet- itive elements.
sex chromosomes Specify an organism’s genetic sex; in humans, the X and Y chromo- somes.
sex-linked disease A disorder due to a mutation in a gene on the X-chromosome; also called X-linked disorder.
SNPs Single Nucleotide Polymorphisms; substitutions in individual bases along a gene or chromosome.
SRY Sex-determining region Y; gene which encodes the testes-determining factor and trig- gers testis development, thus determining sex; located on the Y chromosome.
VNTR Variable Number of Tandem Repeat; short nucleotide sequence ranging from 14 to 100 nucleotides long, organized into clusters of tandem repeats, usually repeated in the range between 4 and 40 times per loci.
inactivation The random inactivation of one X-chromosome in females; occurs early in embryonic development.
How are traits inherited? How about the inheritance of genetic disorders? Are inheri- tance patterns of traits and disorders similar?
Could simple Mendelian inheritance account for such complex traits with vast pheno- typic variation such as height or skin color? What do you think?
Describe the difference between a genetic trait and a genetic disease/disorder.
Define the various modes of inheritance, focusing on the differences between autosomal and sex-linked.
Gives examples of dominant and recessive genetic disorders.
Discuss the inheritance of sex-linked traits.
Discuss complex inheritance patterns.
Define codominant alleles and give examples.
Define incomplete dominance.
Give examples of multiple allele traits.
Discuss how a trisomy condition may be detected.
What is Down syndrome?
List some examples of phenotypes due to abnormal numbers of sex chromosomes.
Discuss the importance of gene therapy.
Describe the most common method of gene therapy.
What is a genetic trait? Is a genetic disease a trait? The answer to these questions may be debated, but a genetic trait is a characteristic of you encoded in your DNA. Could you say
www.ck12.org 418
that a genetic disease is encoded in your DNA? Well, by definition, yes you can.
How are traits inherited? Do different traits have different patterns of inheritance? Is it as simple as a one allele – one phenotype relationship? Or is it more complex? Is there a difference if the gene is located on an autosome or a sex chromosome? Can there be traits due to multiple genes? The answer to all of the above questions is a resounding ‘sometimes.’ Sometimes it is as simple as a one allele – one phenotype relationship, sometimes it is more complex. Sometimes there is a difference depending on the location of the gene. Sometimes traits can be due to multiple genes. Human genetics is an exciting aspect of biology and medicine; an aspect of biology that is extremely important to our health and well being.
Autosomal vs. sex-linked. In terms of genetics, is the location of a gene or trait an important piece of information? Does it make a difference if the gene is located on a sex chromosome or an autosome? It might. Remember from lesson 9.1 that sex chromosomes determine an organism’s sex, so the autosomes are the other chromosomes. Autosomal-linked traits are due to genes on the autosomes; sex-linked traits are due to genes located on the sex chromosomes.
What is the difference between a trait and a genetic disorder? Could a disorder be considered a trait? We tend to think of traits as hair color or skin color and disorders as something that is bad for you. But in terms of genetics, a genetic disorder is a trait. Both may be due to your genes.
How are traits due to genes on autosomes inherited? Autosomal traits due to the effects of one gene are usually inherited in a simple Mendelian pattern. That is, they can be either dominant or recessive. In humans, whereas many genetic disorders are inherited in a recessive manner, simple dominant inheritance accounts for many of a person’s physical characteristics, such as chin, earlobe, hairline and thumb shape. For example, having earlobes that are attached to the head is a recessive trait, whereas heterozygous and homozygous dominant individuals have freely hanging earlobes. If you have a cleft chin, a pointed frontal hairline (called a widow’s peak), or a hitchhiker’s thumb, you have inherited the dominant allele for each characteristic from at least one of your parents. Other dominant traits include the presence of hair on the middle section of your fingers, thick lips, and almond-shaped eyes. A widow’s peak and earlobe shape are displayed in Figure 9.5 and Figure 9.6.

Figure 9.5: A young woman with a widow’s peak, due to a dominant allele. (6)
www.ck12.org 420
Mutations, changes in the DNA or RNA sequence, can have significant phenotypic effects or no effects. We have previously discussed various types of mutations. Now, let’s discuss the outcomes of some of those mutations. As mentioned at the beginning of this chapter, a genetic disorder is a condition caused by abnormalities, such as mutations, in your genes or chromosomes. Genetic disorders are usually present from conception. These disorders include chromosomal abnormalities, in which the individual has too few or too many chro- mosomes or chromosomes with large alterations, or diseases due to a mutation in a specific gene. These defective genes are usually inherited from the parents, hence the term hereditary disease or genetic disorder. Genetic disorders can be inherited in a dominant or recessive manner (Figure 9.7 and Figure 9.8). Recessive disorders require the inheritance of a de- fective gene from each parent. The parents are usually unaffected and are healthy carriers of the defective gene.
How can you, or a geneticist, determine the inheritance pattern of a phenotype? A pedigree, which is essentially a representation of genetic inheritance, is helpful. A pedigree is a chart, much like a family tree, which shows all of the known individuals within a family with a particular phenotype (Table 9.2). Pedigrees have been discussed in the chapter titled Mendelian Genetics. Examples of autosomally inherited disorders include cystic fibrosis, Tay-Sachs disease, phenylketonuria, and achondroplasia.
Table 9.2: Autosomal and Sex-linked Inheritance Patterns
![]()
Inheritance Pattern Description Example
![]()
Autosomal Dominant Only one mutated allele is
needed for a person to be affected by an autosomal dominant disorder. Each affected person usually has one affected parent. There is a 50% chance that a child will inherit the mu- tated gene.
Autosomal Recessive Both copies of the gene must
be mutated for a person to be affected by an autoso- mal recessive disorder. An affected person usually has unaffected parents who each carry a single copy of the mutated gene (and are referred to as carriers).
Huntingtons disease, Achon- droplasia, Neurofibromato- sis 1, Marfan Syndrome, Hereditary nonpolyposis colorectal cancer
Cystic fibrosis, Sickle cell anemia, Tay-Sachs disease, Spinal muscular atrophy
Table 9.2: (continued)
Inheritance Pattern | Description | Example |
X-linked Dominant | X-linked dominant dis- | |
orders are caused by muta- | ||
tions in genes on the X chro- | ||
mosome. Only a few dis- | ||
orders have this inheritance |
pattern.
X-linked Recessive X-linked recessive disor- ders are also caused by mutations in genes on the X chromosome. Males are more frequently affected than females. The sons of a man with an X-linked recessive disorder will not be affected, and his daugh- ters will carry one copy of the mutated gene. A woman who carries an X- linked recessive disorder has a 50% chance of having sons who are affected and a 50% chance of having daughters who carry one copy of the mutated gene.
Y-Linked Y-linked disorders are
caused by mutations on the Y chromosome. Only males can get them, and all of the sons of an affected fa- ther are affected. Y-linked disorders only cause infer- tility, and may be circum- vented with the help of some fertility treatments.
Hemophilia A, Duchenne muscular dystrophy, Color blindness
Male Infertility
![]()
www.ck12.org 422
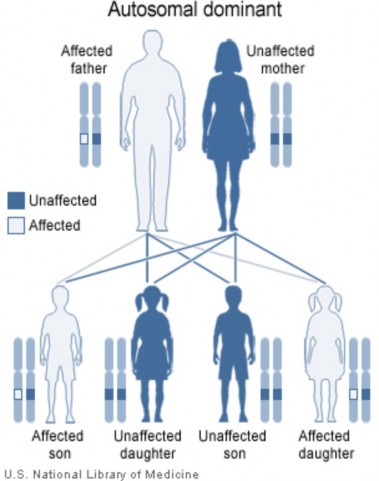
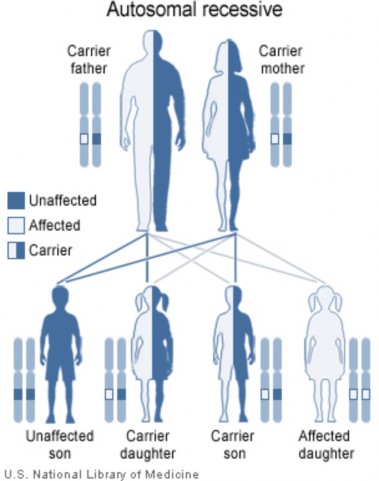
www.ck12.org 424
Cystic fibrosis (CF) is a recessive inheritable disorder caused by a mutation in a gene called the cystic fibrosis transmembrane conductance regulator (CFTR). The product of this gene is a chloride ion channel important in creating sweat, digestive juices, and mucus. Although most people without CF have two working copies of the CFTR gene, only one is needed to prevent cystic fibrosis. CF develops when individuals have a mutation in both copies of the gene, such that neither gene product works normally. CF is one of the most common life shortening diseases. Diagnosis is usually made in childhood. In the United States, approximately 1 in 3,900 children is born with CF (Figure 9.9). One in 22 people of European descent are carriers of a mutated CFTR gene. CF mainly affects the lungs and digestive system, causing difficulty breathing due to thick mucus production, progressive disability, and for some individuals, premature death.
Individuals can be diagnosed prior to birth by genetic testing. Because development of CF in the fetus requires each parent to pass on a mutated copy of the CFTR gene and because CF testing is expensive, testing is often initially performed on just one parent. If that parent is found to be a carrier of a CFTR gene mutation, the other parent is then tested to calculate the risk that their children will have CF. CF can result from more than a thousand different mutations; currently it is not possible to test for each one. As new DNA testing methodologies are developed, testing for more mutations will become more common and less expensive. Testing analyzes DNA for the most common mutations, such as a deletion of amino acid 508 (phenylalenine, also known as ΔF508). If a family has a known uncommon mutation, specific screening for that mutation can be performed. However, it must be noted that because there may be other not yet identified mutations that result in CF, and as not all known mutations are found on current tests, a negative screen does not guarantee that a child will not have CF.
Tay-Sachs disease is a genetic disorder that is fatal in its most common variant, known as Infantile Tay-Sachs disease. Tay-Sachs is an autosomal recessive disorder, requiring the inheritance of a defective gene from each parent. The disease results from the accumulation of harmful quantities of fat in the nerve cells of the brain. Tay-Sachs results from mutations in the HEXA gene located on chromosome 15, which encodes the alpha-subunit of the lysosomal enzyme beta-N-acetylhexosaminidase A, which normally breaks down the fat. More than 90 mutations, including substitutions, insertions, deletions, splice site mutations, and other more complex patterns have been characterized in this gene, and new mutations are still being reported. Each of these mutations alters the protein product, inhibiting the function of the enzyme.
Tay-Sachs disease is a rare disease. Unaffected carriers of a Tay-Sachs allele may not know they have the allele. Other autosomal disorders such as cystic fibrosis and sickle cell anemia
are far more common. The importance of Tay-Sachs lies in the fact that an inexpensive enzyme assay test was developed. The automation of this analysis has provided one of the first ”mass screening” tools in medical genetics. Two unaffected carriers can have a child homozygous for a Tay-Sachs allele, resulting, currently, in a lethal phenotype. Tay-Sachs alleles are maintained in a population through these unknowing heterozygous carriers.
The analysis and screening for Tay-Sachs has became a research and public health model for understanding and preventing all autosomal genetic disorders. Another genetic disease that is easily analyzed in phenylketonuria.
Phenylketonuria (PKU) is an autosomal recessive genetic disorder characterized the in- ability to metabolize the amino acid phenylalanine. PKU is due to a deficiency in the enzyme phenylalanine hydroxylase (PAH). When PAH is deficient, phenylalanine accumulates and is converted into phenylketones, which can be detected in the urine. Left untreated, this con- dition can cause problems with brain development, leading to progressive mental retardation and seizures. However, PKU can be treated with a specific diet, one low in phenylalanine. A diet low in phenylalanine and high in tyrosine can bring about a nearly total cure.
www.ck12.org 426
The incidence of PKU is about 1 in 15,000 live births. In the United States PKU is screened at birth as part of a national biochemical screening program, for every baby born in a hospital. Babies born at home may not be screened. If PKU is diagnosed early enough, an affected newborn can grow up with normal brain development, but only by eating a special diet low in phenylalanine for the rest of his or her life. In essence, this is a protein-free diet. This requires severely restricting or eliminating foods high in protein content (containing phenylalanine), such as breast milk, meat, chicken, fish, nuts, cheese and other dairy products. Starchy foods such as potatoes, bread, pasta, and corn must also be monitored. Many diet foods and diet soft drinks that contain the sweetener aspartame must also be avoided, as aspartame consists of two amino acids: phenylalanine and aspartic acid. Supplementary infant formulas are used in these patients to provide the amino acids and other necessary nutrients that would otherwise be lacking in their diet. Since phenylalanine is required for the synthesis of many proteins, it is necessary to have some of this amino acid, but levels must be strictly controlled. In addition, tyrosine, which is normally derived from phenylalanine, must also be supplemented.
Whereas cystic fibrosis, Tay-Sachs, and phenylketonuria are all autosomal recessive disorders, achondroplasia is an autosomal dominant disorder. Achondroplasia is the most common cause of dwarfism. Achondroplasia is a result of an autosomal dominant mutation in the fibroblast growth factor receptor gene 3 (FGFR3), which causes an abnormality of cartilage formation. FGFR3 normally has a negative regulatory effect on bone growth. In achon- droplasia, the mutated form of the receptor is constitutively active (constantly “turned on”) and this leads to severely shortened bones. Individuals with achondroplasia are heterozygous for the mutation (one mutant copy, one normal copy). Homozygous for the achondroplasia mutation is lethal prior to birth or shortly after birth.
For autosomal dominant disorders, a person with the disorder has a 50% chance of passing on the gene to their offspring. For achondroplasia, this means there will be a 50% chance that each child will have achondroplasia. Since two copies are fatal, if two people with achon- droplasia have a child, there is a 25% chance of the child dying shortly after birth, a 50% chance the child will have achondroplasia, and a 25% chance the child will have a normal phenotype. However, in 3 out of 4 cases, people with achondroplasia are born to parents who don’t have the condition. This is the result of a new mutation. New achondroplasis mutations are associated with increasing paternal age (over 35 years). Studies have demon- strated that new gene mutations are exclusively inherited from the father and occur during spermatogenesis. More than 98% of achondroplasia is caused by a G to A point mutation at nucleotide 1138 of the FGFR3 gene, which causes a glycine to arginine substitution. This makes this particular nucleotide one of the most, if not the most, mutable base in the human genome.
There are three other syndromes with a genetic basis similar to achondroplasia: hypochon-
droplasia, thanatophoric dysplasia, and SADDAN Dysplasia (severe achondroplasia, devel- opmental delay, acanthosis nigricans (a skin condition)). Each of these disorders is also caused by a mutation in the FGFR3 gene. Each of the conditions results in a distinct differ- ence in the degree of severity of the phenotype, with hypochondroplasia having the mildest phenotype. Other genes in which mutations cause a phenotypic spectrum of disease include the collagen genes among others.
Traits controlled by genes located on the sex chromosomes (X and Y) are called sex-linked traits (Figure 9.10). Remember, females have two X chromosomes and males have a X and a Y chromosome. Therefore, any recessive allele on the X chromosome of a male will not be masked by a dominant allele. X-linked traits include the hemophilia and color blindness. Hemophilia is the name of a family of hereditary genetic illnesses that impair the body’s ability to control coagulation. Color Blindness, or color vision deficiency, in humans is the inability to perceive differences between some or all colors that other people can distinguish.
Hemophilia is a group of diseases in which blood does not clot normally. Factors in blood are involved in clotting. Hemophiliacs lacking the normal Factor VIII are said to have Hemophilia A, the most common form. England’s Queen Victoria was a carrier for this disease. The allele was passed to two of her daughters and one son. Since royal families in Europe commonly intermarried, the allele spread, and may have contributed to the downfall of the Russian monarchy.
Genetic red-green color blindness affects men much more often than women, because the genes for the red and green color receptors are located on the X chromosome. Females are red-green color blind only if both of their X chromosomes carry the defective gene, whereas males are color blind if their single X chromosome carries the defective gene. As males have only the one X-chromosome, the gene for red-green color blindness is transmitted from a color blind male to all his daughters, who are usually heterozygous carriers and therefore unaffected. Subsequently, this carrier woman has a fifty percent chance of passing on a X chromosome with a defective gene to each of her male offspring. The sons of an affected male will not inherit the trait from him, since they receive his Y chromosome and not his X chromosome. Should an affected male have children with a carrier or colorblind woman, their daughters may be colorblind by inheriting a X chromosome with the mutant gene from each parent.
Muscular dystrophy is a term encompassing a variety of muscle wasting diseases. The most common type, Duchenne Muscular Dystrophy (DMD), affects cardiac and skeletal muscle, as well as some mental functions. DMD is an X-linked recessive disorder occurring in 1 in 3,500 newborns. Most affected individuals die before their 20th birthday.
www.ck12.org 428
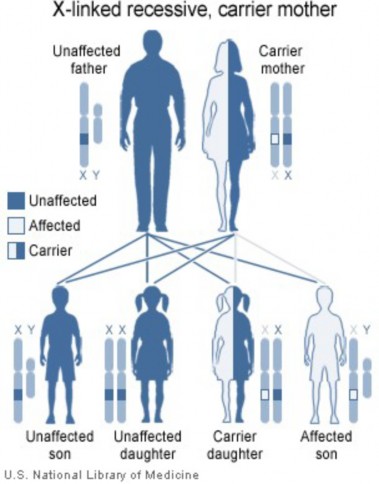
So far we have discussed traits inherited in a simple Mendelian pattern. Either the trait is dominate or recessive. The trait is affected by only one gene. But this is not the case for many genes; rarely is inheritance that simple. More complex patterns of inheritance are common. These were introduced in the chapter titled Mendelian Genetics.
Mendel’s pea plants showed complete dominance of one allele over the other. The off- spring always completely looked like one of the parents – there was never any phenotype “in between” the two parents. The heterozygous individuals were indistinguishable from the homozygous dominant individuals. Is it possible for both alleles to be dominant, or neither to be completely dominant? The answer to both of these questions is yes.
Codominance is when two alleles are both expressed in the heterozygous individual; that is, they both affect the phenotype in separate and distinguishable ways (Figure 9.11). The A, B alleles of the ABO blood group system are a classic example, and these have been discussed in the chapter titled Mendelian Genetics. The A and B alleles are codominant with each other. When a person has both an A and a B allele, the person has type AB blood. When two persons with AB blood type have children, the children can be type A, type B, or type AB. There is a 1A:2AB:1B phenotype ratio instead of the 3:1 phenotype ratio found when one allele is dominant and the other is recessive.
Incomplete dominance is seen in heterozygous individuals with an intermediate pheno- type. For example, if Mendel had ever observed a medium stem length plant when a tall and short plant were crossed, that would have suggested incomplete dominance. In incomplete dominant situations, the phenotype expression is dependent on the dosage of the genes. Two copies of the gene result in full expression, while only one copy produces partial expression and an intermediate phenotype.
Traits controlled by more than two alleles have multiple alleles. Theoretically, any base change will result in a new allele. In fact, within the human population, it may be safe to say that most human genes have more than 2 alleles. Whereas, we think of base changes, or mutations, as resulting in a new phenotype or disease, many base changes result in alleles that do not cause significant change in phenotypes. This is common in collagen genes, for example. The best characterized example of multiple alleles in humans is the ABO blood
www.ck12.org 430
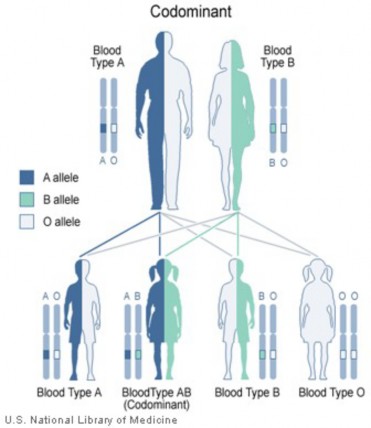
groups, discussed in the chapter titled Mendelian Genetics.
It is easiest to consider situations where each gene affects only one phenotypic characteristic. However, other situations where genes have other effects are common. As mentioned above, multiple alleles resulting in multiple phenotypes are not uncommon.
Many genes have multiple phenotypic effects, a property called pleiotropy. Thus, a new mutation in the gene will affect all the phenotypes/traits associated with the gene simultane- ously. For example, the symptoms associated with sickle-cell disease are due to pleiotropic alleles. Another example is the collagen genes mentioned above. As you will learn later, many bones develop from a cartilage template. This cartilage template is made out of many proteins, with type II collagen, the predominant protein in the cartilage. The gene for this collagen, COL2A1, when mutated, not only affects the skeletal system, but due to its pleiotropic nature, it may also affect a person’s eyes and hearing.
Epistasis is when a gene at one location (locus) alters the phenotypic expression of a gene at another locus. This is seen in the inheritance of coat color in mice. Epistasis takes place when the action of one gene is modified by one or several other genes, which are sometimes called modifier genes. The gene whose phenotype is expressed is said to be epistatic, while the phenotype altered or suppressed is said to be hypostatic.
Polygenic traits are due to the actions of more than one gene and often, their interaction with the environment. These usually result in a measurable range in phenotype, such as height, eye color or skin color. These are known as multifactoral or quantitative character- istics. Polygenic inheritance results in an additive effect of the genes on a single phenotype.
Skin color is a polygenic trait and obviously demonstrates quantitative characteristics. A number of genes factor into determining a person’s natural skin color, so modifying only one of those genes changes the color only slightly. It is currently thought that at least three separately inherited genes contribute to skin pigmentation. Let’s call these three genes A, B, and C. A, B, and C are incompletely dominant to a, b, and c, with A, B, and C each contributing a “unit of darkness” to the phenotype. Therefore an AABBCC individual is very dark, darker than an AaBbCc individual, and much darker than a aabbcc individual. A person may have as many as 6 “dark units” to as few as no “dark units,” and any combination in between. This will result in a phenotypic spectrum of color gradation.
www.ck12.org 432
Many disorders with genetic components are polygenic, including autism, certain cancers, diabetes and numerous others. Most phenotypic characteristics are the result of the interac- tion of multiple genes. The environment plays a significant role in many of these phenotypes. But what happens when multiple genes are either missing or duplicated?
So far we have focused on traits due to one gene or several genes. But what about many genes? What would happen if an entire chromosome were missing or duplicated? What if a human had only 45 chromosomes? Or 47? This real possibility is usually due to mistakes during meiosis; the chromosomes do not fully separate from each other during sperm or egg formation. Specifically, nondisjunction is the failure of replicated chromosomes to separate during anaphase II. If a zygote forms from a gamete lacking a chromosome, a viable embryo cannot be produced. Most human abnormal chromosome numbers result in the death of the developing embryo, often before a woman even realizes she is pregnant. Occasionally, a zygote with an extra chromosome can become a viable embryo and develop.
Trisomy is a state where humans have an extra autosome. That is, they have three of a particular chromosome instead of two. For example, trisomy 18 results from an extra chromosome 18, resulting in 47 total chromosomes. To identify the chromosome number (including an abnormal number), a sample of cells is removed from an individual or devel- oping fetus. Metaphase chromosomes are photographed and a karyotype is produced. A karyotype will display any abnormalities in chromosome number or large chromosomal re- arrangements. Trisomy 8, 9, 12, 13, 16, 18, and 21 have been identified in humans. Trisomy 16 is the most common trisomy in humans, occurring in more than 1% of pregnancies. This condition, however, usually results in spontaneous miscarriage in the first trimester. The most common trisomy in viable births is Trisomy 21.
One of the most common chromosome abnormalities is Down syndrome, due to nondisjunc- tion of chromosome 21 resulting in an extra complete chromosome 21, or part of chromosome 21 (Figure 9.13). Down syndrome is the only autosomal trisomy where an affected individ- ual may survive to adulthood. Individuals with Down syndrome often have some degree of mental retardation, some impairment of physical growth, and a specific facial appearance. With proper assistance, individuals with Down syndrome can become successful, contribut- ing members of society (Figure 9.14). The incidence of Down syndrome increases with maternal age.
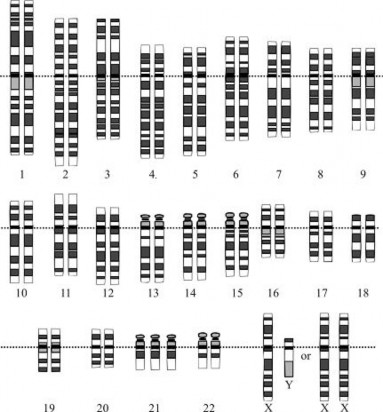
Figure 9.12: Trisomy 21 (Down Syndrome) Karyotype. Note the extra chromosome 21. (3)

Figure 9.13: Child with Down syndrome, exhibiting characteristic facial appearance. (8) www.ck12.org 434

Figure 9.14: Boy with Down Syndrome assembling a bookcase. (10)
What about when a person has more than 2 Y chromosomes, or more than 2 X chromosomes? Or a female with only one X chromosome? Sex-chromosome abnormalities may be caused by nondisjunction of one or more sex chromosomes. Many conditions are known in which there are an abnormal number of sex chromosomes. An X chromosome may be missing (XO), or there may be an extra one (XXX or XXY). There may also be an extra Y chromosome (XYY). Any combination of X and Y chromosomes, as long as there is a Y chromosome, will produce a male (up to XXXXY). These individuals can lead relatively normal lives, but they cannot have children. They may also have some degree of mental retardation. These syndromes include Klinefelter’s syndrome, Turner syndrome and trisomy X.
Klinefelter’s syndrome is caused by the presence of one or more extra copies of the X chromosome in a male’s cells. Extra genetic material from the X chromosome interferes with male sexual development, preventing the testicles from functioning normally and reducing the levels of testosterone. Triple X syndrome (trisomy X) results from an extra copy of the X chromosome in each of a female’s cells. Females with trisomy X have a lower IQ than their siblings. Turner syndrome results when each of a female’s cells has one normal X chromosome and the other sex chromosome is missing or altered. The missing genetic material affects development and causes the characteristic features of the condition, including short stature and infertility.
If someone has a rare genetic disease in her family, can she still have a baby? Is she pre- disposed to pass that phenotype along to her child? These are questions for a professional trained in human genetics. A geneticist and genetic counselor are usually involved in the diagnosis and treatment of human genetic disorders. Families with a genetic disease are referred to a genetic counselor, especially when they wish to determine a baby’s likelihood of inheriting a genetic disease.
Individuals or their families at risk of inheriting a genetic disorder have many questions. What exactly is a genetic disorder? How does a person get it? Can it be passed onto the next generation? Can it be treated? What are the symptoms? Do the symptoms get worse with age? These and many more questions are where a genetic specialist, such as a genetic counselor can help. Genetic counseling is the process by which individuals or their families who are at risk of an inherited disorder, are counseled on many different aspects of the disorder. Genetic counseling may be necessary at any time throughout life, from before pregnancy to adulthood. Before pregnancy, genetic counseling would be appropriate for at risk individuals who are planning a family, such as when one or both individuals are either carriers or have a certain genetic trait. During pregnancy, genetic counseling is necessary for couples if the woman will be over 35 years of age at the time of delivery, if prenatal testing is recommended for any reason, or if an abnormality is noted on an ultrasound or other test. After birth, genetic counseling is appropriate if a birth defect is detected. During childhood, genetic counseling is appropriate if the child manifests any signs of developmental delay or a genetic syndrome, and in adulthood, genetic counseling is appropriate if signs of an adult onset genetic disorder is detected. During genetic counseling, individuals are advised of the consequences and nature of the disorder, the probability of developing or transmitting the disorder, and the options open to them in management and family planning in order to make appropriate decisions. In terms of the actual diagnosis of the disease, molecular analysis may be necessary; this will be discussed in the chapter titled Biotechnology.
”Is it possible to test the developing baby for potential genetic problems? Do you need to remove some of the baby’s DNA? How do you do that?” These questions are appropriate for a geneticist. Sometimes, to make sure the baby is developing properly, prenatal diagnosis is necessary. Prenatal diagnosis refers to the diagnosis of a disease or condition before the baby is born. The reason for prenatal diagnosis is to detect birth defects such as neural tube defects, chromosome abnormalities, genetic diseases and other conditions. It can also be used to determine the sex of the unborn baby, though this can usually be determined by an ultrasonography (ultrasound).
Diagnostic prenatal testing can be by invasive methods or non-invasive methods. Non- invasive methods are much less risky to the patient. Non-invasive methods can only evaluate
www.ck12.org 436
the risk of a condition and cannot actually determine if the fetus has a condition. Non- invasive techniques include examinations of the mother’s womb through ultrasonography and analysis of maternal serum. If an abnormality is indicated by a non-invasive procedure, a more invasive technique may be employed to gather more information. Amniocentesis and chorionic villus sampling (CVS) are invasive procedures. These involve probes or needles that are inserted into the placenta. Amniocentesis can be done from about 14 weeks up to about 20 weeks of the pregnancy and CVS can be done earlier, between 9.5 and 12.5 weeks, but is slightly more risky to the unborn child.
During Amniocentesis a small amount of amniotic fluid, which contains fetal tissues, is extracted from the amnion or amniotic sac surrounding a developing fetus, and the fetal DNA is examined for genetic abnormalities. Amniocentesis is not performed for every pregnancy, but is generally done when an increased risk of genetic defects in the fetus is indicated, by mother’s age (over 35 years is common), family history of genetic defects, or other factors.
Chorionic villus sampling (CVS) involves removing a sample of the chorionic villus (placental tissue) and testing it. It is generally carried out only on pregnant women over the age of 35 and those whose offspring have a higher risk of Down syndrome and other chromosomal conditions. The advantage of CVS is that it can be carried out 10-12 weeks after the last period, earlier than amniocentesis.
DNA from the developing baby may be isolated from either an amniocentesis or CVS. A karyotype may be created from fetal chromosomes following either procedure, or a specific mutation may be analyzed. The analysis of specific mutations will be discussed in the chapter titled Biotechnology.
So, how do you treat genetic disorders? If medically possible, each manifestation can be treated separately. But is there a way to use genetics to treat the root cause of the disease
that is, to fix the mistake in the DNA?
Gene therapy is the insertion of a new gene into an individual’s cells and tissues to treat a disease, replacing a mutant disease-causing allele with a normal, non-mutant allele. Although the technology is still in its early stages of development, it has been used with some success.
There are a number of mechanisms used to replace or repair a defective gene in gene therapy.
A normal gene may be inserted into a nonspecific location within the genome to replace a nonfunctional gene. This approach is most common.
An abnormal gene could be replaced by a normal gene through homologous recombi- nation.
The abnormal gene could be repaired through selective reverse mutation, which returns the gene to its normal, non-mutant state.
The regulation (the degree to which a gene is turned on or off) of a particular gene could be altered.
As stated above, the most common gene therapy approach is to replace a disease-causing allele with a normal allele. To deliver the new allele to the appropriate cells, a carrier, called a vector, must be used. Currently, the most common type of vectors are viruses that have been genetically altered to carry normal human DNA, and not to result in any phenotypes associated with the virus. As viruses have evolved a robust method of delivering their viral genes to human cells, scientists have tried to develop (and are continuing to develop) methods to take advantage of this process, and have these vectors insert human DNA into target cells. Scientists have manipulated the viral genome to remove disease-causing genes and insert therapeutic human genes (Figure 9.15). For obvious reasons, this is currently a field of intense biomedical research.
A patient’s target cells, such as liver or lung cells are infected with the genetically altered virus. The vector then unloads its genetic material containing the therapeutic human gene into the target cell. The generation of a functional protein product from the therapeutic gene should restore the target cell to a normally functioning phenotype. To date, this process has had limited success, but who can say what will happen in the future.
Severe Combined Immunodeficiency, or SCID, is a heritable immunodeficiency - a ge- netic disorder that cripples the immune system. It is also known as the ‘bubble boy’ disease, named after David Vetter, SCID’s most famous patient who lived for over 12 years in a sterilized environment, just like living inside a “bubble.” SCID affects about 1 in 100,000 live births. These babies, if untreated, usually die within one year due to severe, recurrent infections. Treatment options have improved considerably and include bone marrow trans- plants and gene therapy. Children no longer have to live inside a bubble as did David Vetter, who was placed inside his sterile bubble about 10 seconds after birth. He died 15 days after he left his sterile environment, due to an undetected virus in the bone marrow transplant. David was one of the first bone marrow recipients.
More recently gene therapy has proved successful in treating SCID. Insertion of the correct gene into cells of the immune system should correct the problem. Trials started in 1990, and in 1999, the first SCID patients were detected with functional immune systems. Due to some complications these trials had to be stopped, but these issues seem to have been resolved. Gene therapy in individuals with SCID have been human genetics only gene therapy success stories. Since 1999, gene therapy has restored the immune systems of at least seventeen children with the disorder. This raises great hope for other genetic disorders. In your lifetime, it is definitely possible that many genetic disorders may be “cured” by gene therapy. As was mentioned earlier, no one can say what will happen in the future, and no one knows what lies ahead.
www.ck12.org 438
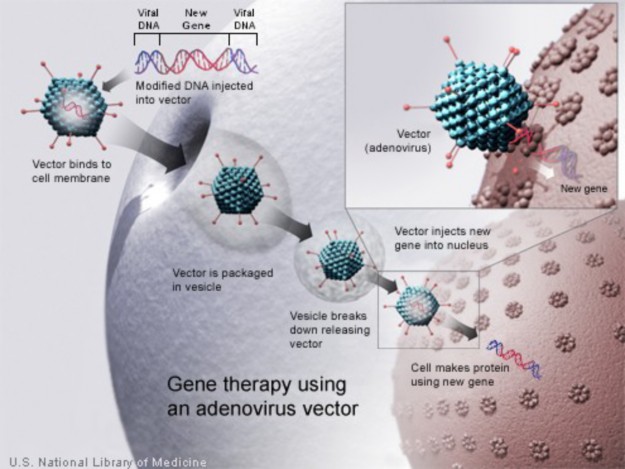
In humans, whereas many genetic disorders are inherited in a recessive manner, simple dominant inheritance accounts for many of a person’s physical characteristics.
Genetic diseases may also be dominantly inherited, such as with achondroplasia.
Genetic diseases may be due to specific mutations within a gene or to large chromoso- mal abnormalities.
Traits controlled by genes located on the sex chromosomes (X and Y) are called sex- linked traits.
Any recessive allele on the X chromosome of a male will not be masked by a dominant allele.
Codominance is when two alleles are both expressed in the heterozygous individual.
Incomplete dominance is seen in heterozygous individuals with an intermediate phe- notype.
Traits controlled by more than two alleles have multiple alleles.
Many genes have multiple phenotypic effects, a property called pleiotropy.
Epistasis is when a gene at one location (locus) alters the phenotypic expression of a gene at another locus.
Polygenic traits are due to the actions of more than one gene and often, their interaction with the environment.
Trisomy is a state where humans have an extra autosome; they have three of a partic- ular chromosome instead of two.
The most common trisomy in viable births is Trisomy 21 (Down Syndrome).
Prenatal diagnosis refers to the diagnosis of a disease or condition before the baby is born.
Amniocentesis and choronic villus sampling are invasive methods involved in prenatal diagnosis.
Gene therapy is the insertion of a new gene into an individual’s cells and tissues to treat a disease, replacing a mutant disease-causing allele with a normal, non-mutant allele.
What is a genetic disease?
Discuss the main difference between autosomal and sex-linked.
Why is variation within the human genome important?
Why is it more common for males to have X-linked disorders?
Describe how a mutation can lead to a genetic disease.
Discuss how a new mutation can become a new dominant allele.
How are autosomal traits usually inherited? Give examples of traits.
How are genetic diseases usually inherited? Are there exceptions? Give examples.
Discuss the difference between codominance and incomplete dominance. Give exam- www.ck12.org 440
ples.
What is meant by trisomy? (Beginning) How can trisomy phenotypes be detected?
What is the most common viable trisomy disorder?
List conditions involving an abnormal number of sex chromosomes.
Why is genetic counseling important?
What is gene therapy?
Describe the most common approach to gene therapy.
The National Human Genome research Institute:
The Dolan DNA Learning Center:
DNA Interactive:
A Science Odyssey: DNA Workshop:
Kimball’s Biology Pages:
achondroplasia An autosomal dominant disorder; the most common cause of dwarfism.
amniocentesis A prenatal diagnostic procedure in which a small amount of amniotic fluid, which contains fetal tissues, is extracted from the amnion or amniotic sac surrounding a developing fetus, so that the fetal DNA is examined for genetic abnormalities.
autosomal dominant disorder A disorder in which only one mutated allele is needed for a person to be affected.
autosomal recessive disorder A disorder in which both copies of the gene must be mu- tated for a person to be affected.
autosome Any chromosome other than a sex chromosome.
chorionic villus sampling (CVS) A prenatal diagnostic procedure which involves re- moving a sample of the chorionic villus (placental tissue) and testing it.
codominance When two alleles are both expressed in the heterozygous individual; both alleles affect the phenotype in separate and distinguishable ways.
cystic fibrosis (CF) A recessive inheritable disorder caused by a mutation in a gene called the cystic fibrosis transmembrane conductance regulator (CFTR).
Duchenne muscular dystrophy (DMD) The most common type of muscular dystro- phy; an X-linked recessive disorder.
epistasis When a gene at one location (locus) alters the phenotypic expression of a gene at another locus.
gene therapy The insertion of a new gene into an individual’s cells and tissues to treat a disease, replacing a mutant disease-causing allele with a normal, non-mutant allele.
genetic counseling The process by which individuals or their families who are at risk of an inherited disorder are counseled on many different aspects of the disorder.
genetic counselor An individual trained in human genetics and counseling.
hemophilia A group of diseases in which blood does not clot normally.
incomplete dominance Occurs in heterozygous individuals with an intermediate pheno- type; neither allele is dominant over the other.
Klinefelter’s syndrome Caused by the presence of one or more extra copies of the X chromosome in a male’s cells.
multiple allele traits Traits controlled by more than two alleles.
muscular dystrophy A term encompassing a variety of muscle wasting diseases.
mutation A change in the nucleotide sequence of DNA or RNA.
nondisjunction The failure of replicated chromosomes to separate during anaphase II of meiosis.
pedigree A chart which shows all of the known individuals within a family with a particular phenotype; a representation of genetic inheritance.
www.ck12.org 442
Phenylketonuria (PKU) An autosomal recessive genetic disorder characterized the in- ability to metabolize the amino acid phenylalanine.
pleiotropy Having multiple phenotypic effects.
polygenic traits Traits that are due to the actions of more than one gene and often, their interaction with the environment.
prenatal diagnosis The diagnosis of a disease or condition in a developing baby; done before the baby is born.
Severe Combined Immunodeficiency (SCID) A heritable immunodeficiency; a genetic disorder that cripples the immune system.
sex chromosomes Specify an organism’s genetic sex; in humans, the X and Y chromo- somes.
sex-linked traits Traits controlled by genes located on the sex chromosomes (X and Y).
Tay-Sachs disease An autosomal recessive genetic disorder that is fatal in early childhood; results from the accumulation of harmful quantities of fat in the nerve cells of the brain.
trisomy A state where humans have an extra autosome, having 47 chromosomes instead of 46. For example, trisomy 16 results in a third chromosome 16.
trisomy 21 Down syndrome; individuals often have some degree of mental retardation, some impairment of physical growth, and a specific facial appearance.
trisomy X Triple X syndrome; results from an extra copy of the X chromosome in each of a female’s cells.
linked disorder A disorder caused by a mutation in a gene on the X chromosome; may be dominant or recessive, though the majority of X-linked disorders are recessive.
Y-linked disorder A disorder caused by a mutation in a gene on the Y chromosome; only affects males.
In this chapter, we discussed human genetics as involved in human health. In the next chapter, we will discuss biotechnology. With gene therapy, we can see how biotechnology will play a significant role in society’s future.
Can you speculate on the role of biotechnology in our future?
What other roles for biotechnology do you envision?
Why is biotechnology important?
NASA. http://en.wikipedia.org/wiki/Image:Earlobes_free_attached.jpg. Public Domain.
NIH. http://commons.wikimedia.org/wiki/Image:Autorecessive.jpg. Public Domain.
http://commons.wikimedia.org/wiki/Image:Down_Syndrome_Karyotype.png. Public Domain.
NIH. http://en.wikipedia.org/wiki/File:XlinkRecessive.jpg. Public Domain.
http://commons.wikimedia.org/wiki/File:CFtreatmentvest2.JPG. GNU-FDL.
http://en.wikipedia.org/wiki/Image:Human_genome_to_genes.png. CC-BY 2.0.
Erin Ryan. http://commons.wikimedia.org/wiki/Image:Brushfield_eyes.jpg. Public Domain.
NIH. http://commons.wikimedia.org/wiki/Image:Codominant.jpg. Public Domain.
Rob Kay. Boy with Down Syndrome assembling a bookcase.. GNU-FDL.
NIH. http://commons.wikimedia.org/wiki/Image:Autodominant.jpg. Public Domain.
http://www.genome.gov/Pages/Hyperion/DIR/VIP/Glossary/Illustration/ karyotype.cfm?key=karyotype. Public Domain.
http://www.genome.gov/Pages/Hyperion/DIR/VIP/Glossary/Illustration/ chromosome.cfm?key=chromosome. Public Domain.
www.ck12.org 444
sex_chromosomes.cfm?key=sex%20chromosome . Public Domain.
NIH. http://en.wikipedia.org/wiki/Image:Gene_therapy.jpg. Public Domain.
www.ck12.org 446
What is meant by DNA technology?
What is the Human Genome Project?
Describe the goals of the Human Genome Project.
Describe gene cloning and the processes involved.
What is PCR?
Describe the processes involved in PCR.
Is it really possible to clone people? Another question is, should we clone people? Are scien- tific fantasies, such as depicted on TV shows such as Star Trek or in the movie GATTACA, actually a possibility? Who can really say? How, really, will science affect our future? The answers partially lie in the field of biotechnology.
Biotechnology is technology based on biological applications. These applications are in- creasingly used in medicine, agriculture and food science. Biotechnology combines many features of biology, including genetics, molecular biology, biochemistry, embryology, and cell biology. Many aspects of biotechnology center around DNA and its applications, other- wise known as DNA technology. We could devote a whole textbook to current applications of biotechnology; in this chapter, however, we will focus on the applications towards medicine and the extension into the forensic sciences. First, though, we need to understand DNA technology.
What is DNA technology? Is it using and manipulating DNA to help people? Is it using DNA to make better medicines and individualized treatments? Is it analyzing DNA to determine predispositions to genetic diseases? The answers to these questions, and many more, is yes. And the answers to many of these issues begin with the Human Genome Project.
If we are all 99.9% genetically identical, what makes us different? How does that 0.1% make us tall or short, light or dark, develop cancer or not? To understand that 0.1%, we also need to understand the other 99.9%. Understanding the human genome is the goal of The Human Genome Project (HGP). This project, publicly funded by the United States Department of Energy (DOE) (Figure 10.1); and the National Human Genome Research Institute (NHGRI), part of the National Institutes of Health (NIH), may be one of the landmark scientific events of our lifetime. Our Molecular Selves video discusses the human genome, and is available at http://www.genome.gov/25520211.

Figure 10.1: The Human Genome Project logo of the DOE. (1)
The goal of the HGP is to understand the genetic make-up of the human species by deter- www.ck12.org 448
mining the DNA sequence of the human genome (Figure 10.2);and the genome of a few model organisms. However, it is not just determining the 3 billion bases; it is understanding what they mean. Today, all 3 billion base pairs have been sequenced, and the genes in that sequence are in the process of being identified and characterized. A preliminary estimate of the number of genes in the human genome is around 22,000 to 23,000.
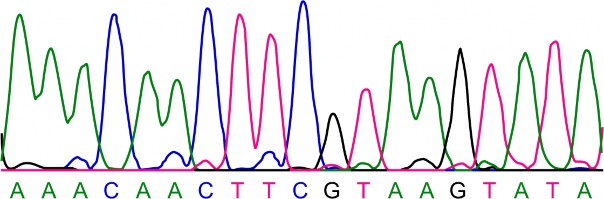
The sequence of the human DNA is stored in databases available to anyone on the Inter- net. The U.S. National Center for Biotechnology Information (NCBI), part of the NIH, as well as comparable organizations in Europe and Japan, maintain the genomic sequences in a database known as Genbank. Protein sequences are also maintained in this database. The sequences in these databases are the combined sequences of anonymous donors, and as such do not yet address the individual differences that make us unique. However, the known sequence does lay the foundation to identify the unique differences among all of us. Most of the currently identified variations among individuals will be single nucleotide polymor- phisms, or SNPs. A SNP (pronounced ”snip”) is a DNA sequence variation occurring at a single nucleotide in the genome. For example, two sequenced DNA fragments from different individuals, GGATCTA to GGATTTA, contain a difference in a single nucleotide. If this base change occurs in a gene, the base change then results in two alleles: the C allele and the T allele. Remember an allele is an alternative form of a gene. Almost all common SNPs have only two alleles. The effect of these SNPs on protein structure and function, and any effect on the resulting phenotype, is an extensive field of study.
You probably have heard of cloning. Whereas cloning of humans has many ethical issues associated with it, the cloning of genes has been ongoing for well over 30 years, with cloning
of animals occurring more recently. Gene cloning, also known as molecular cloning, refers to the process of isolating a DNA sequence of interest for the purpose of making multiple copies of it. The identical copies are clones. In 1973, Stanley Cohen and Herbert Boyer developed techniques to make recombinant DNA, a form of artificial DNA.
Recombinant DNA is engineered through the combination of two or more DNA strands, combining DNA sequences which would not normally occur together. In other words, se- lected DNA (or the DNA of ”interest”) is inserted into an existing organismal genome, such as a bacterial plasmid DNA or some other sort of vector. The recombinant DNA can then be inserted into another cell, such as a bacterial cell, for amplification and possibly production of the resulting protein. This process is called transformation, the genetic alteration of a cell resulting from the uptake, incorporation, and expression of foreign genetic material. Recombinant DNA technology was made possible by the discovery of restriction endonucle- ases.
In the classical restriction enzyme digestion and ligation cloning protocols, cloning of any DNA fragment essentially involves four steps:
isolation of the DNA of interest (or target DNA)
ligation
transfection (or transformation)
a screening/selection procedure.
For an overview of cloning, see http://www.hhmi.org/biointeractive/media/DNAi_genetic_ eng-sm.mov.
Initially, the DNA fragment to be cloned needs to be isolated. This DNA of interest may be a gene, part of a gene, a promoter, or another segment of DNA, and is frequently isolated by the Polymerase Chain Reaction (PCR) or restriction enzyme digestion. A restriction enzyme (or restriction endonuclease) is an enzyme that cuts double-stranded DNA at a specific sequence (Table 10.1). The enzyme makes two incisions, one through each strand of the double helix, without damaging the nitrogenous bases. This produces either overlapping ends (also known as sticky ends) or blunt ends.
![]()
![]()
A. G↓AATTC B. CCC↓GGG CTTAA↑G GGG↑CCC
![]()
www.ck12.org 450
A. EcoRI digestion produces overlapping ”sticky” ends: The enzyme cleaves between the G and A on both strands. B. SmaII restriction enzyme cleavage produces ”blunt” ends. The enzyme cleaves between the G and C on both strands.
(Source: Created by: Doug Wilkin, License: CC-BY-SA)
The 1978 Nobel Prize in Medicine was awarded to Daniel Nathans and Hamilton Smith for the discovery of restriction endonucleases. The first practical use of their work was the manipulation of E. coli bacteria to produce human insulin for diabetics.
Once the DNA of interest is isolated, a ligation procedure is necessary to insert the amplified fragment into a vector to produce the recombinant DNA molecule. Restriction fragments (or a fragment and a plasmid/vector) can be spliced together, provided their ends are com- plementary. Blunt end ligation is also possible.
The plasmid or vector (which is usually circular) is digested with restriction enzymes, opening up the vector to allow insertion of the target DNA. The two DNAs are then incubated with DNA ligase, an enzyme that can attach together strands of DNA with double strand breaks. This produces the recombinant DNA molecule. Figure 10.3 depicts a plasmid with two additional segments of DNA ligated into the plasmid, producing the recombinant DNA molecule. Figure 10.4 depicts DNA before and after ligation.
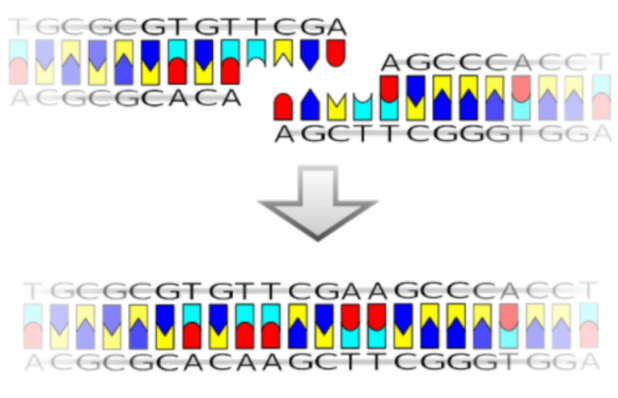
www.ck12.org 452
Following ligation, the recombinant DNA is placed into a host cell, usually bacterial, in a process called transfection or transformation. Finally, the transfected cells are cultured. Many of these cultures may not contain a plasmid with the target DNA as the transfection process is not usually 100% successful, so the appropriate cultures with the DNA of interest must be selected. Many plasmids/vectors include selectable markers - usually some sort of antibiotic resistance (Figure 10.3). When cultures are grown in the presence of an antibiotic, only bacteria transfected with the vector containing resistance to that antibiotic should grow. However, these selection procedures do not guarantee that the DNA of insert is present in the cells. Further analysis of the resulting colonies is required to confirm that cloning was successful. This may be accomplished by means of a process known as PCR (see below) or restriction fragment analysis, both of which need to be followed by gel electrophoresis and/or DNA sequencing (DNA sequence analysis).
DNA sequence analysis (the analysis of the order of the nitrogenous bases that make up the DNA), PCR, or restriction fragment analysis will all determine if the plasmid/vector contains the insert. Restriction fragment analysis is digestion of isolated plasmid/vector DNA with restriction enzymes. If the isolated DNA contains the target DNA, that fragment will be excised by the restriction enzyme digestion. Gel electrophoresis will separate DNA molecules based on size and charge. Examples are shown in Figure 10.5.
Gel electrophoresis is an analytical technique used to separate DNA fragments by size and charge. Notice in Figure 10.5 that the ”gels” are rectangular in shape. The gels are made of a gelatin-like material of either agarose or polyacrylamide. An electric field, with a positive charge applied at one end of the gel, and a negative charge at the other end, forces the fragments to migrate through the gel. DNA molecules migrate from negative to positive charges due to the net negative charge of the phosphate groups in the DNA backbone. Longer molecules migrate more slowly through the gel matrix. After the separation is completed, DNA fragments of different lengths can be visualized using a fluorescent dye specific for DNA, such as ethidium bromide. The resulting stained gel shows bands correspond to DNA molecules of different lengths, which also correspond to different molecular weights. Band size is usually determined by comparison to DNA ladders containing DNA fragments of known length. Gel electrophoresis can also be used to separate RNA molecules and proteins.
The Polymerase Chain Reaction (PCR) is used to amplify specific regions of a DNA strand millions of times. A region may be a number of loci, a single gene, a part of a gene, or a non-coding sequence. This technique produces a useful quantity of DNA for analysis,
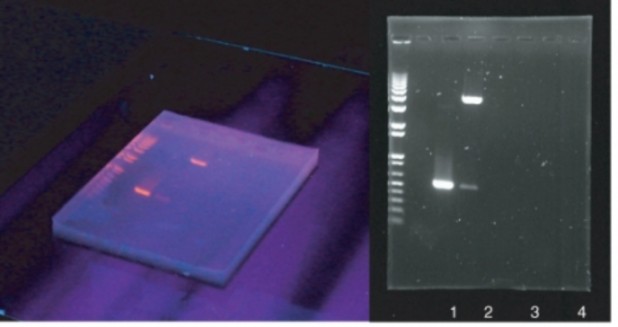
www.ck12.org 454
be it medical, forensic or some other form of analysis. Amplification of DNA from as little as a single cell is possible. Whole genome amplification is also possible.
PCR utilizes a heat stable DNA polymerase, Taq polymerase, named after the thermophilic bacterium Thermus aquaticus, from which it was originally isolated. T. aquaticus is a bac- terium that lives in hot springs and hydrothermal vents, and Taq polymerase is able to withstand the high temperatures required to denature DNA during PCR (discussed below). Taq polymerase’s optimum temperature for activity is between 75°C and 80°C. Recently other DNA polymerases have also been used for PCR.
A basic PCR involves a series of repeating cycles involving three main steps (see Figure
10.6):
denaturation of the double stranded DNA
annealing of specific oligonucleotide primers
extension of the primers to amplify the region of DNA of interest
These steps will be discussed in additional detail below.
The oligonucleotide primers are single stranded pieces of DNA that correspond to the 5’ and 3’ ends of the DNA region to be amplified. These primers will anneal to the correspond- ing segment of denatured DNA. Taq Polymerase, in the presence of free deoxynucleotide triphosphates (dNTPs), will extend the primers to create double stranded DNA. After many cycles of denaturation, annealing and extension, the region between the two primers will be amplified.
The PCR is commonly carried out in a thermal cycler, a machine that automatically allows heating and cooling of the reactions to control the temperature required at each reaction step (see below). The PCR usually consists of a series of about 30 to 35 cycles. Most commonly, PCR is carried out in three repeating steps, with some modifications for the first and last step.
PCR is usually performed in small tubes or wells in a tray, each often beginning with the complete genome of the species being studied. As only a specific sequence from that genome is of interest, the sequence specific primers are targeted to that sequence. PCR is done with all the building blocks necessary to create DNA: template DNA, primers, dNTPs, and a polymerase.
The three basic steps of PCR (Figure 10.6) are:
Denaturation step: This step is the first regular cycling event and consists of heating the reaction to 94 - 98°C for 30 to 60 seconds. It disrupts the hydrogen bonds between complementary bases of the DNA strands, yielding single strands of DNA.
Annealing step: The reaction temperature is lowered to 50-65°C for 30 to 60 seconds, allowing annealing of the primers to the single-stranded DNA template. Stable hy- drogen bonds form between the DNA strand (the template) and the primers when the
primer sequence very closely matches the complementary template sequence. Primers are usually 17 - 22 nucleotides long and are carefully designed to bind to only one site in the genome. The polymerase binds to the primer-template hybrid and begins DNA synthesis.
Extension step: A temperature of around 72°C is used for this step, which is close to the optimum temperature of Taq polymerase. At this step the Taq polymerase extends the primer by adding dNTPs, using one DNA strand as a template to create a the other (new) DNA strand. The extension time depends on the length of the DNA fragment to be amplified. As a standard, at its optimum temperature, the DNA polymerase will polymerize a thousand bases in one minute.
Utilizing the PCR, DNA can be amplified millions of times to generate quantities of DNA that can be used for a number of purposes. These include the use of DNA for prenatal or genetic testing, such as testing for a specific mutation. PCR has revolutionized the fields of biotechnology, human genetics, and a number of other sciences. Many of the applications will be discussed in the following lesson. PCR was developed in 1983 by Kary Mullis. Due to the importance of this process and the significance it has had on scientific research, Dr. Mullis was awarded the Nobel Prize in Chemistry in 1993, just 10 years after his discovery.
To say that PCR, molecular cloning and the Human Genome Project has revolutionized biology and medicine would be an understatement. These efforts have led to numerous acco- lades, including Nobel prizes, and more may follow. Some of the ways that these discoveries have shaped our lives are the focus of the next lesson.
Biotechnology is technology based on biological applications, combining many features of Biology including genetics, molecular biology, biochemistry, embryology, and cell biology.
The goal of the Human Genome Project is to understand the genetic make-up of the human species by determining the DNA sequence of the human genome and the genome of a few model organisms.
Gene cloning, also known as molecular cloning, refers to the process of isolating a DNA sequence of interest for the purpose of making multiple copies of it (cloning).
Classic gene cloning involves the following steps:
Restriction enzyme digestion and ligation
Isolation of DNA
Ligation
Transfection and Selection
Gel electrophoresis
www.ck12.org 456
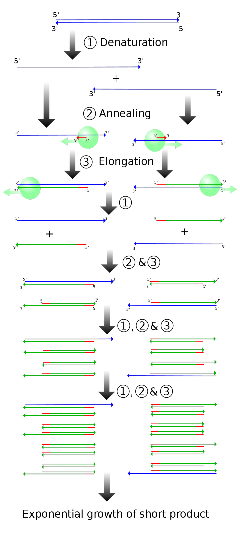
The Polymerase Chain Reaction (PCR) is used to amplify millions of times specific regions of a DNA strand. This can be a number of loci, a single gene, a part of a gene, or a non-coding sequence.
PCR usually involves the following steps:
Denaturation step
Annealing step
Extension step
Why are biotechnology and DNA technology considered the same?
What are the goals of the Human Genome Project?
Is the DNA sequence information generated by the HGP available to anyone, and if so, how?
How are gene cloning and recombinant DNA related?
Describe the process of gene cloning.
How does gel electrophoresis analyze DNA?
What is PCR?
What allows PCR to be done at high temperatures?
Describe the PCR process.
http://www.ornl.gov/sci/techresources/Human_Genome/home.shtml
http://croptechnology.unl.edu/viewLesson.cgi?LessonID=957884601
http://nobelprize.org/educational_games/chemistry/pcr/index.html
http://www.vtaide.com/png/cloning.htm
biotechnology Technology based on biological applications.
gel electrophoresis An analytical technique used to separate DNA fragments by size and charge.
www.ck12.org 458
Genbank The U.S. National Center for Biotechnology Information, part of the National Institutes of Health, which maintains the genomic sequences in a database.
gene cloning The process of isolating a DNA sequence of interest for the purpose of mak- ing multiple copies of it.
The Human Genome Project A project to understand the genetic make-up of the hu- man species by determining the DNA sequence of the human genome and the genome of a few model organisms.
plasmid (or vector) A small circular piece of DNA that carries the recombinant DNA into a host organism for cloning.
polymerase chain reaction (PCR) A repeating series of cycles used to amplify millions of times specific regions of a DNA strand.
recombinant DNA Engineered through the combination of two or more DNA strands that combine DNA sequences which would not normally occur together.
restriction enzyme (or restriction endonuclease) An enzyme that cuts double-stranded DNA.
Taq polymerase Named after the thermophilic bacterium Thermus aquaticus from which it was originally isolated, is the heat-stable polymerase used in the PCR reaction.
The Human Genome Project, gene cloning, and PCR are some of the most remarkable scientific achievements of the recent past. But how can these milestones make our lives better?
Medicine and food science are just two of the categories that benefit from biotechnology. Speculate on how our lives are made better by these achievements.
Describe various applications of biotechnology as related to medicine, agriculture and forensic science.
How is DNA technology related to genetic testing and prenatal diagnosis?
Why is biotechnology so important in agriculture?
Why is DNA analysis the most important tool of the forensic scientist?
Describe forensic STR analysis.
Discuss some of the ELSI associated with biotechnology.
Scientists have sequenced a consensus version of the human genome. Now what? Do we know what all the genes are or what they do? Not yet. Do we know what phenotypes are associated with mutations in the genes? For many genes, or even most genes, we do not. Do we even know exactly how many genes we have? Not exactly. And we are far away from knowing what makes us all unique. So how does this information help us? The Human Genome Project has been labeled a landmark scientific event. But what can we do with this information?
There are many applications of genetic information, including applications in medicine and agriculture. The applications of genetics to forensic science have become one of the most important aspects of the criminal justice system. And of course, these applications raise many ethical questions. These applications and questions will be the focus of this lesson.
As discussed in the first lesson of this chapter, the Human Genome Project has opened up many applications to take advantage of what we know about our genome in order to help us. Many of these applications are medically related. Others will be legally related. And yet still other uses of DNA technology include those in agriculture and the food sciences.
Understanding and curing genetic diseases is the ultimate goal of human geneticists. As discussed in the Human Genetics chapter, gene therapy is the insertion of a new gene into an individual’s cells and tissues to treat a disease, replacing a mutant disease-causing allele with a normal, non-mutant allele. Of course, the findings of the Human Genome Project are significant in determining the disease-causing alleles.
In the 1920s, there was no known way to produce insulin, which was needed by people to remove excess sugar from the bloodstream. People with diabetes either lack insulin, produce low levels of insulin, or are resistant to insulin, and thus they may need external insulin to control blood glucose levels. This problem was solved, at least temporarily, when it was found that insulin from a pig’s pancreas could be used in humans. This method was the primary solution for diabetes until recently. The problem with insulin production was raised again: there were not enough pigs to provide the quantities of insulin needed. Scientists needed to devise another way. This led to one of the biggest breakthroughs in recombinant DNA technology: the cloning of the human insulin gene.
www.ck12.org 460
By methods discussed in the first lesson in this chapter, the specific gene sequence that codes for human insulin was introduced into the bacteria E. coli. The transformed gene altered the genetic makeup of the bacterial cells, such that in a 24 hour period, billions of E. coli containing the human insulin gene resulted, producing human insulin to be administered to patients.
Though the production of human insulin by recombinant DNA procedures is an extremely significant event, many other aspects of DNA technology are beginning to become reality. In medicine, modern biotechnology provides significant applications in such areas as pharma- cogenomics, genetic testing (and prenatal diagnosis), and gene therapy. These applications use our knowledge of biology to improve our health and our lives. Many of these medical applications are based on the findings of the Human Genome Project.
Currently, millions of individuals with high cholesterol take a similar type of drug. You may know of people who take a medicine to help with their cholesterol levels. However, these drugs probably work slightly differently in many of those people. In some, it lowers their cholesterol significantly; in others it may lower it only moderately; and in some, it may have no effect at all. Why the difference? Because of the genetic background of all people. Pharmacogenomics, a combination of pharmacology and genomics (the study of the genome) that refers to the study of the relationship between pharmaceuticals and genetics, may explain and simplify this problem.
Pharmacogenomics is the study of how the genetic inheritance of an individual affects his or her body’s response to drugs. In other words, pharmacogenomics will lead to the design and production of drugs that are adapted to each person’s genetic makeup.
Pharmacogenomics will result in the following benefits:
Development of tailor-made medicines. Using pharmacogenomics, pharmaceutical companies will be able to create drugs based on the proteins, enzymes and RNA molecules that are associated with specific genes and diseases. These tailor-made drugs promise not only to maximize the beneficial effects of the medicine, but also to decrease damage to nearby healthy cells.
More accurate methods of determining appropriate drug dosages. Knowing a patient’s genetics will enable doctors to determine how well his or her body can process and metabolize a medicine. This will allow doctors to prescribe the proper levels of the medicine, allowing the medicine to have optimal results.
Improvements in the drug discovery and approval process. Once the genes and proteins associated with a disease are known, the discovery of new medicines will be made easier using these genes and proteins as targets for the medicine. In addition to creating much more beneficial medicines, this could significantly shorten the drug discovery process.
Better vaccines. Safer vaccines can be designed and produced by organisms trans- formed with DNA sequences from an antigen. These vaccines will trigger the immune response without the risks of infection. They will be capable of being engineered to carry several strains of pathogen at once, combining several vaccines into one.
Let’s propose a hypothetical situation: unfortunately, your family is predisposed to develop a genetic disease. You and your spouse want to have a baby, but you want to know the likelihood of the child developing the disease.
This scenario could happen to anyone. As we learn more and more about disease causing genes, it will become easier to test for mutations in those genes. Currently, is there any way to determine if a baby will develop a disease due to a known mutation? Is it possible to screen for a mutation in a developing baby? Yes.
Genetic testing involves the direct examination of DNA sequences. A scientist scans, by any number of methods, a patient’s DNA for mutated sequences. Genetic testing can be used to:
Diagnose a disease.
Confirm a diagnosis.
Provide information about the course of a disease.
Confirm the existence of a disease.
Predict the risk of future development of a disease in otherwise healthy individuals or their children.
Identify carriers (unaffected individuals who are heterozygous for a recessive disease gene).
Perform prenatal diagnostic screening.
Perform newborn screening.
Consultations with human geneticists and genetic counselors are an important first step in genetic testing. They will most likely prescribe some sort of prenatal screening (see the Human Genetics chapter). Prenatal screening (also known as prenatal diagnosis or testing) is the testing for diseases or conditions in a fetus or embryo before it is born. Methods may involve amniocentesis or chorionic villus sampling to remove fetal cells. DNA can be isolated from these cells and analyzed. If the mutation that results in the phenotype is known, that specific mutation can be tested, either through restriction fragment length polymorphism analysis or, more likely, through PCR and DNA sequence analysis. As it is the baby’s DNA that is being analyzed, the analysis will determine if the developing baby will have the mutation and develop the phenotype, or not have the mutation. Parents can then be informed of the probability of the baby developing the disease.
www.ck12.org 462
In human genetics, preimplantation genetic diagnosis (PIGD) is genetic analysis per- formed on embryos prior to implantation. PIGD is considered an alternative to prenatal diagnosis. Its main advantage is that it avoids selective pregnancy termination, as the method makes it highly likely that the baby will be free of the disease in question. In PIGD, in vitro fertilization is used to obtain embryos for analysis. DNA is isolated from developing embryos prior to implantation, and specific genetic loci are screened for mutations, usually using PCR based analysis. Embryos that lack the specific mutation can then be implanted into the mother, thereby guaranteeing that the developing baby will not have the specific mutation analyzed for (and thus not have the disease associated with that mutation).
Biotechnology has many other useful applications besides those that are medically related. Many of these are in agriculture and food science. These include the development of trans- genic crops - the placement of genes into plants to give the crop a beneficial trait. Benefits include:
Improved yield from crops.
Reduced vulnerability of crops to environmental stresses.
Increased nutritional qualities of food crops.
Improved taste, texture or appearance of food.
Reduced dependence on fertilizers, pesticides and other agrochemicals.
Production of vaccines.
Using biotechnology techniques, one or two genes may be transferred into a crop to give a new trait to that crop. This is done in the hope of increasing its yield. However, these increases in yield have proved to be difficult to achieve. Current genetic engineering techniques work best for single gene effects - that is traits inherited in a simple Mendelian fashion. Many of the genetic characteristics associated with crop yield, such as enhanced growth, are controlled by a large number of genes, each of which just has a slight effect on the overall yield. There is, therefore, still much research, including genetic research, to be done in this area.
Crops are obviously dependent on environmental conditions. Drought can destroy crop yields, as can too much rain or floods. But what if crops could be developed to withstand these harsh conditions? Biotechnology will allow the development of crops containing genes that will enable them to withstand biotic and abiotic stresses. For example, drought and excessively salty soil are two significant factors affecting crop productivity. But there are
crops that can withstand these harsh conditions. Why? Probably because of that plant’s genetics. So biotechnologists are studying plants that can cope with these extreme condi- tions, trying to identify and isolate the genes that control these beneficial traits. The genes could then be transferred into more desirable crops, with the hope of producing the same phenotypes in those crops.
Thale cress (Figure 10.7), a species of Arabidopsis (Arabidopsis thaliana), is a tiny weed that is often used for plant research because it is very easy to grow and its genome has been extensively characterized. Scientists have identified a gene from this plant, At-DBF2, that confers resistance to some environmental stresses. When this gene is inserted into tomato and tobacco cells, the cells were able to withstand environmental stresses like salt, drought, cold and heat far better than ordinary cells. If these preliminary results prove successful in larger trials, then At-DBF2 genes could help in engineering crops that can better withstand harsh environments. Researchers have also created transgenic rice plants that are resistant to rice yellow mottle virus (RYMV). In Africa, this virus destroys much of the rice crops and makes the surviving plants more susceptible to fungal infections.

Figure 10.7: Thale cress. (11)
Maybe you’ve heard over and over that eating beans is good for you. True? Well, maybe. But what if it were possible to increase the nutritional qualities of food? One would think that would be beneficial to society. So, can biotechnology be used to do just that? Scientists
www.ck12.org 464
are working on modifying proteins in foods to increase their nutritional qualities. Also, proteins in legumes and cereals may be transformed to provide all the amino acids needed by human beings for a balanced diet.
Have you ever gone to the grocery store, bought some fruit and never gotten around to eating it? Maybe you haven’t, but I bet your parents have. Modern biotechnology can be used to slow down the process of spoilage so that fruit can ripen longer on the plant and then be transported to the consumer with a still reasonable shelf life. This is extremely important in parts of the world where time from harvest to the consumer may be longer than in other areas. In addition to improving the taste, texture and appearance of fruit, it will also extend the usable life of the fruit. As the world population grows and grows, this may become a fairly important issue. Extending the life of fruit can expand the market for farmers in developing countries due to the reduction in spoilage. This has successfully been demonstrated in the tomato. The first genetically modified food product was a tomato which was transformed to delay its ripening. Researchers in Indonesia, Malaysia, Thailand, Philippines and Vietnam are currently working on delayed-ripening papayas.
There is growing concern regarding the use of pesticides in agriculture. Therefore, many of the current commercial applications of modern biotechnology in agriculture are focused on reducing the dependence of farmers on these chemicals. For example, Bacillus thuringiensis (Bt) is a soil bacterium that produces a protein that can act as an insecticide, known as the Bt toxin. But it is a protein, not a foreign chemical. Could this protein be used in crops instead of pesticides? Traditionally, an insecticidal spray has been produced from these bacteria. As a spray, the Bt toxin is in an inactive state and requires digestion by an insect to become active and have any effect. Crop plants have now been engineered to contain and express the genes for the Bt toxin, which they produce in its active form. When an insect ingests the transgenic crop, it stops feeding and soon thereafter dies as a result of the Bt toxin binding to its gut wall. Bt corn is now commercially available in a number of countries to control corn borer (a lepidopteran insect like moths and butterflies), which is otherwise controlled by insecticidal spraying.
In addition to insects, weeds have also been a menace to farmers - just ask anyone with a garden how much they hate weeds. They can quickly compete for water and nutrients needed by other plants. Sure, farmers can use herbicides to kill weeds, but do these chemicals also harm the crops? Can biotechnology help with this issue? Some crops have also been genetically engineered to acquire tolerance to the herbicides - allowing the crops to grow, but killing the weeds. But the lack of cost effective herbicides with a broad range of activity - that

Figure 10.8: Kenyans examining genetically modified insect resistant transgenic Bt corn. (12)
www.ck12.org 466
do not harm crops - is a problem in weed management. Multiple applications of numerous herbicides are routinely needed to control the wide range of weeds that are harmful to crops. And at times these herbicides are being used as a preventive measure – that is, spraying to prevent weeds from developing rather than spraying after weeds form. So these chemicals are being added to crops. This practice is followed by mechanical and/or hand weeding to control weeds that are not controlled by the chemicals. Crops that are tolerant of herbicides would obviously be a tremendous benefit to farmers (Figure 10.8). The introduction of herbicide tolerant crops has the potential to reduce the number of chemicals needed during a growing season, thereby increasing crop yield due to improved weed management and decreased harm to the crops.
In 2001, 626,000 square kilometers of transgenic crops were planted. Seventy-seven percent of the transgenic crops were developed for herbicide tolerance in soybean, corn, and cotton, 15% were Bt crops for insect resistance, and 8% were developed with genes for both insect resistance and herbicide tolerance in cotton and corn.
Many little children hate shots. And many children in parts of the world do not even have access to vaccines. But what if these vaccines were available in an edible form? Modern biotechnology is increasingly being applied for novel uses other than food. Banana trees and tomato plants have been genetically engineered to produce vaccines in their fruit. If future clinical trials prove successful, the advantages of edible vaccines would be enormous, especially for developing countries. The transgenic plants could be grown locally and cheaply. Edible vaccines would not require the use of syringes, which, in addition to being unpleasant, can be a source of infections if contaminated.
DNA technology has proved very beneficial to humans. Transgenic animals are animals that have incorporated a gene from another species into their genome (Figure 10.9). They are used as experimental models to perform phenotypic tests with genes whose function is unknown, or to generate animals that are susceptible to certain compounds or stresses for testing purposes. Other applications include the production of human hormones, such as insulin. Many times these animals are rodents, such as mice, or fruit flies (Drosophila melanogaster). Fruit flies are extremely useful as genetic models to study the effects of genetic changes on development.
But transgenic animals just have one novel gene. What about a whole new genome? It could be argued that human cloning is one of the techniques of modern biotechnology. It involves the removal of the nucleus from one cell and its placement in an unfertilized egg cell whose nucleus has either been deactivated or removed. Theoretically this would result

www.ck12.org 468
in an individual genetically identical to the donor. Of course, there are many ethical issues associated with human cloning. But animal cloning is arguably a different story.
In February 1997, Ian Wilmut and his colleagues at the Roslin Institute announced the successful cloning of a sheep named Dolly from the mammary glands of an adult female (Figure 10.10). Dolly was the first mammal to be cloned from an adult somatic cell. The cloning of Dolly made it apparent to many that the techniques used to produce her could someday be used to clone human beings. This resulted in tremendous controversy because of its ethical implications. After cloning was successfully demonstrated by Dolly’s creators, many other large mammals, including horses and bulls, were cloned. Cloning is now considered a promising tool for preserving endangered species.

In animal cloning, the nucleus from a somatic cell is inserted into an egg cell in which the nucleus has been removed. The resulting cell is cultivated and after a few divisions, the egg cell is placed into a uterus where it is allowed to develop into a fetus that is genetically
identical to the donor of the original nucleus (Figure 10.11). For an animation of cloning, see http://www.dnalc.org/resources/animations/cloning101.html.
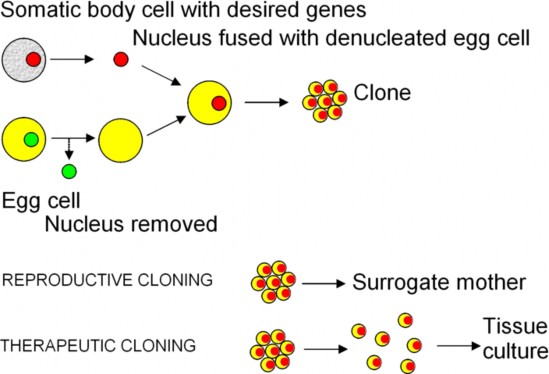
You know that DNA can be used to distinguish individuals from each other. You may have heard that DNA can also be used to match evidence and suspects and help solve crimes. This is demonstrated on shows like CSI: Crime Scene Investigation. But how is this done? How is a “genetic fingerprint,” a DNA pattern unique to each individual (except identical twins) created? Genetic fingerprinting, or DNA fingerprinting, distinguishes between individuals of the same species using only samples of their DNA. DNA fingerprinting has thus become one of the most powerful tools of the forensic scientist, enabling law enforcement personnel to match biological evidence from crime scenes to suspects. As any two humans have the majority of their DNA sequence in common, those sequences which demonstrate high variability must be analyzed. This DNA analysis was first developed using DNA hy- bridization techniques, but now is almost exclusively PCR-based.
www.ck12.org 470
DNA fingerprinting was developed by Sir Alec Jeffreys in 1985. Genetic fingerprinting ex- ploits highly variable repeating sequences. Two categories of these sequences are microsatel- lites and minisatellites. Microsatellites, also known as short tandem repeats (STRs), consist of adjacent repeating units of 2 - 10 bases in length, for example (GATC)n, where GATC is a tetranucleotide (4 base) repeat and n refers to the number of repeats. It is the number of repeating units at a given locus that is variable. An STR profile can be created for any individual by analyzing a series of STRs (Figure 10.12). Two unrelated humans will be unlikely to have the same numbers of repeats at a given locus.
In STR profiling, PCR is used to obtain enough DNA to then detect the number of repeats at 13 specific loci. PCR products are separated by gel or capillary electrophoresis. By examining enough STR loci and counting how many repeats of a specific STR sequence there are at a given locus, it is possible to create a unique genetic profile of an individual. STR analysis has become the prevalent analysis method for determining genetic profiles in forensic cases. It is possible to establish a match that is extremely unlikely to have arisen by coincidence, except in the case of identical twins, who will have identical genetic profiles. The polymorphisms (different in the number of repeats) displayed at each STR region will be shared by approximately 5 - 20% of individuals. When analyzing STRs at multiple loci, such as the 13 STRs analyzed in forensic DNA analysis, it is the unique combinations of these polymorphisms in an individual that makes this method unmatched as an identification tool. The more STR regions that are analyzed in an individual the more discriminating the test becomes.
Capillary electrophoresis is similar to gel electrophoresis but uses a capillary tube filled with the gelatin material.
Genetic fingerprinting is used in forensic science to match suspects to samples of blood, hair, saliva or semen, or other sources of DNA. It has also led to several exonerations of formerly convicted suspects. Genetic fingerprinting is also used for identifying human remains, testing for paternity, matching organ donors, studying populations of wild animals, and establishing the province or composition of foods. It has also been used to generate hypotheses on the pattern of the human migration.
In the United States, DNA fingerprint profiles generated from the 13 STR loci are stored in CODIS, The Combined DNA Index System, maintained by the Federal Bureau of In- vestigation. As of 2007, CODIS maintained over 4.5 million profiles. Profiles maintained in CODIS are compiled from both suspects and evidence, and therefore are used to help solve criminal cases. Profiles of missing persons are also maintained in CODIS. The true power of STR analysis is in its statistical power of discrimination. Because the 13 loci are indepen- dently assorted, the laws of probabilities can be applied. This means that if someone has the genotype of ABC at three independent loci, then the probability of having that specific genotype is the probability of having type A times the probability of having type B times the probability of having type C. This has resulted in the ability to generate match probabilities of 1 in a quintillion (1 with 18 zeros after it) or more, that is, the chance of two samples matching by coincidence is greater than the number of people on the planet, or the number
of people that have ever lived!
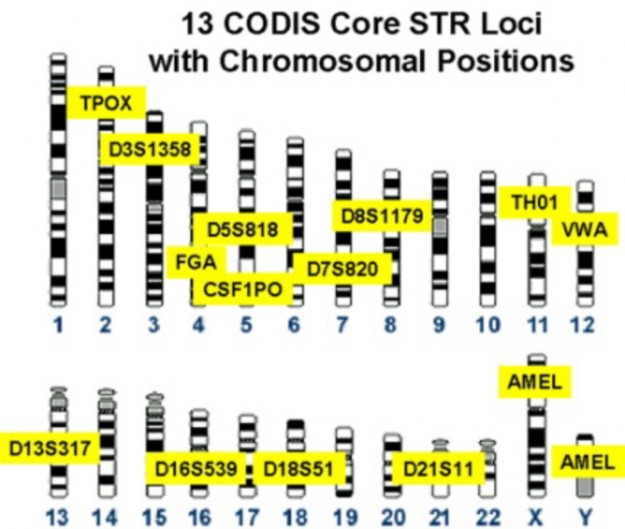
The development of PCR has enabled STR analysis to become the method of choice for DNA identification. Prior to PCR, other methods were utilized. These include restriction fragment length polymorphism (RFLP) analysis and Southern blot analysis.
Prior to the development of PCR, restriction enzyme digestion of DNA followed by Southern blot analysis was used for DNA fingerprinting. This analysis is based on the polymorphic nature of restriction enzyme sites among different individuals, hence restriction fragment
www.ck12.org 472
length polymorphisms are formed after digestion of DNA with these enzymes. A South- ern blot, named after its inventor Edwin Southern, is a method used to check for the presence of a specific DNA sequence in a DNA sample. Once an individual’s DNA is di- gested with a specific restriction enzyme, the resulting fragments are analyzed by Southern blot analysis. These fragments will produce a specific pattern for that individual. Southern blotting is also used for other molecular biology procedures, including gene identification and isolation. Other blotting methods that employ similar principles have been developed. These include the western blot and northern blot. These procedures analyze proteins and RNA respectively.
RFLP and Southern blot analysis involved several steps:
First, the DNA being analyzed is cut into different-sized pieces using restriction en- zymes.
The resulting DNA fragments are separated by gel electrophoresis.
Next, an alkaline solution or heat is applied to the gel so that the DNA denatures and separates into single strands.
Nitrocellulose paper is pressed evenly against the gel and then baked so the DNA is permanently attached to it. The DNA is now ready to be analyzed using a radioactive single-stranded DNA probe in a hybridization reaction.
After hybridization, excess probe is washed from the membrane, and the pattern of hybridization is visualized on X-ray film by autoradiography (Figure 10.13).
Hybridization is when two genetic sequences bind together because of the hydrogen bonds that form between the base pairs. To make hybridization work, the radioactive probe has to be denatured so that it is single-stranded. The denatured probe and the Southern blot are incubated together, allowing the probe to bind to the corresponding fragment on the Southern blot. The probe will bond to the denatured DNA wherever it finds a fit. Hy- bridization of a probe made to a variable segment of DNA will produce a DNA fingerprint pattern specific for an individual. This procedure has a number of steps and is very labor intensive. PCR-based methods are much simpler.
Imagine someone analyzes part of your DNA. Who controls that information? What if your health insurance company found out you were predisposed to develop a devastating genetic disease. Might they decide to cancel your insurance?
Privacy issues concerning genetic information is a growing issue in this day and age, especially among those who donate DNA for large-scale sequence-variation studies. Other concerns have been to anticipate how the resulting data may affect concepts of race and ethnicity; identify potential uses (or misuses) of genetic data in workplaces, schools, and courts; identify
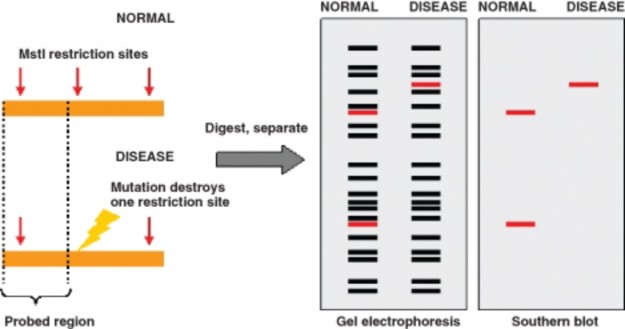
commercial uses; and foresee impacts of genetic advances on the concepts of humanity and personal responsibility.
ELSI stands for Ethical, Legal and Social Issues. It’s a term associated with the Human Genome project. This project didn’t only have the goal to identify all the approximately 20,000 – 24,000 genes in the human genome, but also to address the ELSI that might arise from the project. The U.S. Department of Energy (DOE) and the National Human Genome Research Institute (NHGRI) of the National Institutes of Health (NIH) devoted 3% to 5% of their annual human genome research budget toward studying ethical, legal, and social issues surrounding the availability of your genetic information. This represents the world’s largest bioethics program and has become a model for ELSI programs around the world.
Rapid advances in DNA-based research, human genetics, and their applications have resulted in new and complex ethical and legal issues for society. ELSI programs that identify and address these implications have been an integral part of the Human Genome Project since its inception. These programs have resulted in a body of work that promotes education and helps guide the conduct of genetic research and the development of related medical and public policies.
ELSI programs address the following issues, among others:
Privacy and confidentiality issues concerning personal genetic information. www.ck12.org 474
The fairness in the use of personal genetic information by insurers, employers, courts, schools, adoption agencies, and the military, among others.
The psychological impact and stigmatization due to an individual’s genetic differences.
Clinical issues. These include the education of doctors and other health service providers, patients, and the general public in the capabilities and uses of genetic information, and the scientific/medical limitations of genetic testing. Clinical issues also include the im- plementation of standards and quality-control measures in genetic testing procedures.
Reproductive issues. These include adequate informed consent for complex and poten- tially controversial procedures, and the use of genetic information in making decisions concerning reproductive options.
Uncertainties associated with genetic testing. The current and future uncertainties associated with testing for susceptibilities to a genetic condition raise many ethical issues, as does the testing for predisposition to a complex condition (such as heart disease) linked to multiple genes and gene-environment interactions.
Health and environmental issues concerning genetically modified foods and microbes.
Commercialization of genetic products including property rights, such as patents and copyrights, and issues concerning the accessibility to genetic data and materials.
Biotechnology will have a tremendous impact on our future - of this there is no doubt. Is society entering some dangerous areas? Well, many of these issues have never been ana- lyzed until now. With the discovery of countless amounts of genetic information and the development of its applications, many questions need to be addressed.
Who should have access to personal genetic information, and how will it be used?
Who owns and controls genetic information?
How does personal genetic information affect an individual and society’s perceptions of that individual?
How does genomic information affect members of minority communities?
How reliable and useful is fetal genetic testing?
How will genetic tests be evaluated and regulated for accuracy, reliability, and utility?
How do we prepare the public to make informed choices?
Should testing be performed when no treatment is available?
Should parents have the right to have their minor children tested for adult-onset dis- eases?
Are genetic tests reliable and interpretable by the medical community?
Where is the line between medical treatment and enhancement?
Are genetically modified foods and other products safe for humans and the environ- ment?
How will these technologies affect developing nations’ dependence on the West?
Who owns genes and other pieces of DNA?
Will patenting DNA sequences limit their accessibility and development into useful products?
Are scientific fantasies, such as those depicted on TV shows such as Star Trek or in the movie GATTACA, a possibility? Who can really say? How, really, will biotechnology affect our future? It seems as if the possibilities are endless.
In medicine, modern biotechnology provides significant applications in such areas as pharmacogenomics, genetic testing (prenatal diagnosis), and gene therapy.
Pharmacogenomics, the combination of pharmacology and genomics, is the study of the relationship between pharmaceuticals and genetics.
Pharmacogenomics will result in the following benefits:
Development of tailor-made medicines.
More accurate methods of determining appropriate drug dosages.
Improvements in the drug discovery and approval process.
Better vaccines.
Genetic testing involves the direct examination of DNA sequences.
Genetic testing can be used to: diagnose a disease; confirm a diagnosis; provide prog- nostic information about the course of a disease; confirm the existence of a disease; predict the risk of future development of a disease in otherwise healthy individuals or their children; screen for carriers (unaffected individuals who are heterozygous for a disease gene); perform prenatal diagnostic screening; and perform newborn screening.
Biotechnology in agriculture includes the development of transgenic crops - the place- ment of genes into plants to give the crop a beneficial trait. Benefits include improved yield from crops, reduced vulnerability of crops to environmental stresses, increased nutritional qualities of food crops, improved taste, texture or appearance of food, re- duced dependence on fertilizers, pesticides and other agrochemicals, and production of vaccines.
Transgenic animals are animals that have incorporated a gene from another species into their genome. They are used as experimental models to perform phenotypic tests with genes whose function is unknown, or to generate animals that are susceptible to certain compounds or stresses for testing purposes. Other applications include the production of human hormones, such as insulin.
Animal cloning is the generation of genetically identical animals using DNA from a donor animal, not a gamete. Dolly, a sheep, was the first mammal to be cloned from an adult somatic cell.
Genetic fingerprinting, or DNA fingerprinting, distinguishes between individuals of the same species using only samples of their DNA. DNA fingerprinting has thus become one of the most powerful tools of the forensic scientist, enabling law enforcement personnel to match biological evidence from crime scenes to suspects.
www.ck12.org 476
ELSI stands for Ethical, Legal and Social Issues. This is a term associated with the Human Genome project. Rapid advances in DNA-based research, human genetics, and their applications have resulted in new and complex ethical and legal issues for society. ELSI programs that identify and address these implications have been an integral part of the Human Genome Project since its inception. These programs have resulted in a body of work that promotes education and helps guide the conduct of genetic research and the development of related medical and public policies.
List applications of DNA technology.
List how DNA technology is used in agriculture.
How is DNA technology used in medicine?
What are some of the benefits of pharmacogenomics?
Describe how pharmacogenomics will result in specialty medicines.
What are potential uses of genetic testing?
Describe how DNA technology can improve yield from crops.
Describe how DNA technology can be used to reduce vulnerability to environmental stresses. Why is this important? State an example.
What is the difference between a transgenic animal and a cloned animal?
Who was Dolly? Why was she important?
What is a DNA fingerprint and how is it used?
What is STR profiling?
Describe why ELSI programs are important.
List some ELSI issues.
http://www.dna.gov/basics/http://www.ornl.gov/sci/techresources/Human_Genome/ medicine/pharma.shtml
http://www.ornl.gov/sci/techresources/Human_Genome/medicine/pharma.shtml
http://www.ornl.gov/sci/techresources/Human_Genome/medicine/genetherapy. shtml
http://www.americanheart.org/presenter.jhtml?identifier=4566
http://www.ornl.gov/sci/techresources/Human_Genome/elsi/forensics.shtml
http://en.wikipedia.org/wiki/Main_Page
CODIS The Combined DNA Index System, is maintained by the Federal Bureau of In- vestigation and stores DNA profiles.
ELSI Ethical, Legal and Social Issues. This term is associated with the Human Genome Project.
genetic fingerprinting (DNA fingerprinting) Creates a unique DNA pattern that dis- tinguishes between individuals of the same species using only samples of their DNA.
genetic testing The direct examination of DNA sequences for mutated sequence.
microsatellites (short tandem repeats) Adjacent repeating units of 2 - 10 bases in length, for example (GATC)n, where GATC is a tetranucleotide repeat and n refers to the number of repeats.
pharmacogenomics The combination of pharmacology and genomics, is the study of the relationship between pharmaceuticals and genetics. It is the study of how the genetic inheritance of an individual affects his or her body’s response to drugs.
preimplantation genetic diagnosis (PGD) Genetic analysis performed on embryos prior to implantation.
prenatal diagnosis (prenatal screening) Testing for diseases or conditions in a fetus or embryo before it is born. Methods may involve amniocentesis or chorionic villus sampling to remove fetal cells.
restriction fragment length polymorphism (RFLP) Analysis that analyzes the dif- ferences between restriction enzyme sites.
southern blot Named after its inventor Edwin Southern, is a method used to check for the presence of a specific DNA sequence in a DNA sample.
www.ck12.org 478
STR profiling Analyzes 13 STR loci to create a DNA profile utilized in forensic analysis.
transgenic animals Animals that have incorporated a gene from another species into their genome.
transgenic crops The result of placement of genes into plants to give the crop a beneficial trait.
We have spent the past few chapters discussing genetics, molecular biology, and their impli- cations. These are implicitly related to evolution.
Can you hypothesize on the relationship between genetics and evolution?
Why is an understanding of the principals of DNA and inheritance essential to under- stand evolution?
The Human Genome Project logo of the DOE.. Public Domain.
http://en.wikipedia.org/wiki/Image:Dolly_the_sheep2-thumb.jpg.
http://commons.wikimedia.org/wiki/Image:Ligation.svg. GNU-FDL.
http://en.wikipedia.org/wiki/File:Agarosegelphoto.jpg. GNU-FDL.
http://commons.wikimedia.org/wiki/Image:DNA_sequence.png. Public Domain.
http://www.ncbi.nlm.nih.gov/projects/genome/probe/doc/TechRFLP.shtml. Public Domain.
http://en.wikipedia.org/wiki/Image:Codis_profile.jpg. Public Domain.
http://en.wikipedia.org/wiki/File:GloFish.jpg. The photographer of this work allows anyone to use it for any purpose including unrestricted redistribution, commercial use, and modification.
http://commons.wikimedia.org/wiki/Image:Example_plasmid.png. GNU-FDL.
Thale cress.. GNU-FDL.
http://commons.wikimedia.org/wiki/File:Btcornafrica.jpg. CC-BY 2.5.
http://commons.wikimedia.org/wiki/Image:Cloning_diagram_english.png. GNU-FDL.
www.ck12.org 480
Use the conditions required for fossilization to explain why fossils are rare.
List and give examples of different types of fossils.
Discuss the way in which index fossils contribute to our understanding of the history of life.
Compare relative dating of fossils and rock layers to absolute dating.
Explain why “carbon dating” is an inadequate description of aging rocks and fossils.
Describe how molecular clocks clarify evolutionary relationships.
Compare and contrast Geologic Time with absolute time. Include limits of each.
Sequence the levels of organization of the Geologic Time Scale from largest to smallest.
Arrange the four major Eons and one Supereon from youngest to oldest.
Describe and interpret the differences in fossil abundance throughout the Geologic Time Scale.
Distinguish macroevolution from microevolution and explain their relationship.
Describe the general pattern of the fossil record to support Darwin’s idea that all life descended from a common ancestor.
Evaluate the role of mass extinctions and episodic speciation in evolution.
Identify types of major environmental change in the earth’s history and relate them to patterns in the fossil record.
Analyze ways in which the Geologic Time Scale may give false impressions of the history of life.
Discuss rates of macroevolution and speciation, comparing and contrasting the ideas of gradualism, punctuated equilibrium and quantum evolution.
Compare and contrast adaptive radiation (divergent evolution) to convergent evolution.
Indicate some changes in geography which influence evolution.
Use patterns of evolution and environmental change to account for worldwide differ- ences in the distribution of mammals (placentals vs. marsupials).
Define and give examples of coevolution.
“There is grandeur in this view of life, with its several powers, having been originally breathed into a few forms or into one; and that, whilst this planet has gone cycling on according to the fixed law of gravity, from so simple a beginning endless forms most beautiful and most wonderful have been, and are being, evolved.” - Charles Darwin, Origin of Species. 1859
The history of life as we currently understand it is vast and wondrous and dramatic and humbling and ennobling. Vast is the almost unimaginable expanse of time during which life has flourished: four billion years is our current best estimate! Wondrous is the diversity of species throughout that time: some 30 million species occupy Earth today, and over 90% of all which have ever lived are extinct. Dramatic are the tales of change in environment and in diversity: ice ages, volcanism, continental drift, mass extinction, and bursts of evolutionary creativity have all shaped the natural environment. Humbling is the recognition that humans have played a relatively small part in the history; if the 4.6 billion-year story of Earth is reduced to a single cosmological day, humans occupy just the last minute and a half, and civilization covers less than the final second (Figure 11.1). Ennobling is the story’s revelation that we are related to and interdependent with all other species – back 4 billion years to “so simple a beginning” (Figure 11.25). Finally, the history of life suggests we might add one more striking impression: Terrifying is the realization that we are in many ways unique among species in our unprecedented power to change the environment, influence evolution, and destroy life’s diversity.
If we as a species occupy just the last minute and one-half of the cosmological day, how can we know the vast history of that 4.6 billion-year “day?” How did we arrive at 4.6 billion years as the age of the earth? How we know is the topic of this first lesson on the history of life.
By age three, you probably knew that dinosaurs are part of the history of life. Our under- standing of where they belong in the tale is relatively recent, but “dragon bones” have been known for thousands of years in China and Europe. Fossils are preserved remains or traces of organisms that provide extremely rare but vivid windows to the past. Because most parts of organisms decompose rapidly following death, fossilization is an exceptionally uncommon
www.ck12.org 482
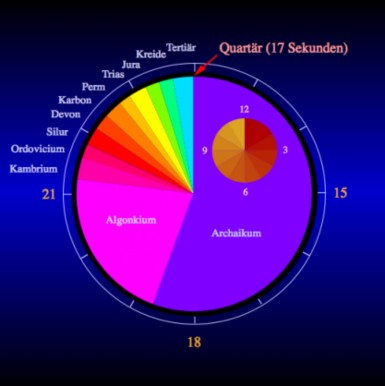
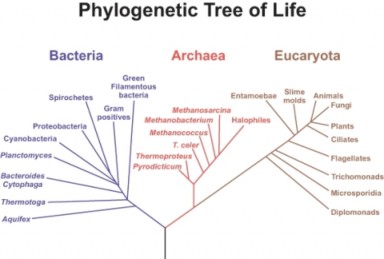
occurrence, and usually preserves only hard body parts, shown in Figure 11.3. Remains must be covered by sediment almost immediately. Buried organisms may experience min- eralization (occasionally even within cells), or they may decay, leaving a space within the sediment later replaced with rock. Alternative pathways to fossilization include freezing, drying, trapping in resin (amber) or burial in anoxic (oxygen-free) environments. Trace fossils preserve footprints, burrows, droppings, eggs, nests, and other types of impressions. Overall, a great variety of types of fossils reveal the history of life, shown in Figure 11.4.
Images in rock tell us what kinds of organisms lived in the past, but the story of life cannot be told without knowing when various organisms appeared. Paleontologists use two methods to date fossils. The oldest method looks at position within a sedimentary column of rock to give a fossil’s relative age. If the rock layers are undisturbed, the deepest layers are the oldest, and layers near the surface are the youngest, shown in Figure 11.5. Widespread, short-lived index fossils can help identify rock layers of the same age spread around the earth, shown in Figure 11.6. Combining worldwide observations of relative position and composition resulted in a Geologic Time Scale for the Earth – a column of rock layers which reflects the history of sedimentary rock formation and changing life, stretching back to a time which apparently held no life. The fossil record showed patterns which, combined with his observations of living species, led Charles Darwin to conclude that all life on Earth descended from a single, simple common ancestor.
Relative age, however, only begins the story. Absolute aging, also known as absolute dating, uses radioactive isotopes, whose known half-lives can be used to calculate the
www.ck12.org 484

Figure 11.3: Scipionyx samniticus was a small dinosaur from Early Cretaceous Italy. This fossil of a juvenile only a few inches long is considered one of the most important vertebrate fossils ever discovered, because unlike most, it preserved internal organs as well as hard structures. Fossilization of an organism is itself a very rare event; preservation of soft tissues is even less likely. (16)
number of years which have elapsed since a rock formed. Radioactive decay is a random but exponential process. An isotope’s half life gives the time period over which half of the material will decay to a different, relatively stable product, shown in Figure 11.7. The ratio of the original isotope to its decay product changes in a predictable way. This predictability allows the relative abundances of isotope and decay product to be used as a clock that measures the time from the incorporation of the isotope into a rock or a fossil to the present.
For example, half of a sample of Carbon-14 will decay to Nitrogen in 5,370 years. Cosmic rays cause the formation of Carbon-14 from the more common and stable Carbon-12 at a relatively constant rate, so carbon dioxide in the atmosphere contains relatively constant, predictable amounts. Organisms acquire carbon from various mechanisms – plants from CO2, and animals and other heterotrophs through food chains. When an organism dies, its carbon intake stops, and existing Carbon-14 atoms decay exponentially, according to their 5,370-year half-life. The proportion of Carbon-14 in the organism’s remains indicates the time lapsed since its death.
Table 11.1: Isotopes Used to Measure Absolute Age of Rocks and Fossils
Isotope | Decay Product | Half-life | Aging of Rocks or Fossils |
Carbon-14 Uranium 238/235 | Nitrogen 5370 years Thorium/Protactinium80,000/34,300 ye | Up to 60,000 years ars Hundreds of thou- sands of years | |
485 | |||
Table 11.1: (continued)
![]()
Isotope Decay Product Half-life Aging of Rocks or
Fossils
![]()
Potassium-40 Argon 1.3 billion years Earth’s oldest rocks
Uranium-238/235 Lead 4.5 billion /704 mil- lion years
1 million to > 4.5 billion years
![]()
Carbon-14 has a relatively short half-life, so its use for absolute dating is limited to a max- imum of about 60,000 years. Other isotopes are used to reach deeper into geological time. Uranium-238 and Uranium-235 decay to different isotopes of lead with half-lives of 4.46 bil- lion and 704 million years, respectively, and together allow dating of rocks between 1 million and over 4.5 billion years old. Table 11.1 shows some of the many isotopes can be used to study rocks throughout Earth’s 4.6 billion year history.
Absolute aging techniques confirmed and brought into focus the rock layer story geologists and paleontologists had developed with relative dating. They pushed Earth’s history back
4.6 billion years, and showed that complex life evolved after some two billion years in which bacteria alone populated the Earth.
Further confirmation of common ancestry included molecular clocks, which measure changes in DNA or proteins to indicate degrees of relationship among species. Comparing DNA se- quences of several species of primates, for example, shows that chimpanzees are more closely related to humans than are gorillas or baboons, shown in Table 11.2. If we assume uniform rates of mutation, we can estimate not only degree of relationship, but time back to common ancestry. Because DNA sequences (and mutations) determine the sequence of amino acids in proteins, Hemoglobin and other proteins are also used as “clocks.” Both DNA and protein clocks support a universal common ancestor for life, confirming the story which continues to unfold as new discoveries expand the fossil record. Molecular clocks, together with evidence from the fossil record, allows scientists to estimate how long ago various groups of organisms diverged evolutionarily from one another.
Table 11.2: DNA “Clock” Comparison of Primates
![]()
Species % Difference in Nucleotide Sequence, Com- pared to Humans
![]()
Human 0
Chimpanzee 1.2
Gorilla 1.6
Baboon 6.6
![]()
www.ck12.org 486


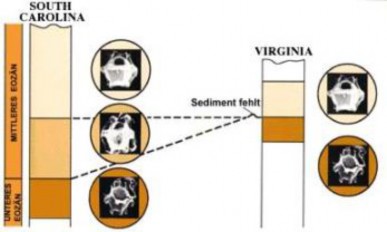
www.ck12.org 488
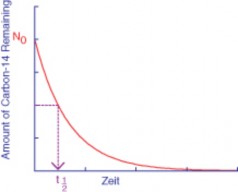
We noted in the previous section that observation of rock layers, dating techniques, and correlation of similar strata from around the world led to the development of a Geologic Time Scale (Figure 11.8). How does the scale divide 4.6 billion years of history? What themes emerge from its stories of the past?
One theme is almost unimaginable amounts of time. The deep time of Earth’s history is far beyond our experience, and our knowledge is far more detailed for recent millennia than for the distant past. A scale divided into evenly spaced periods of time would not show that detail. Instead, Geologic Time Scale divisions mark major events which highlight changes in climate, geography, atmosphere, and life. The largest units of time are Eons. Eons include smaller Eras, which in turn include Periods, Epochs, and Stages. Faunal stages identify specific fossil groups. Terms such as Upper/Late and Lower/Early divide parts of the scale into more recent and more distant subunits.
Four eons comprise the history of Earth, and their names refer to a second major theme of Earth history: the evolution of life. The Phanerozoic (“visible life”) Eon spans the most recent 545 million years and includes three Eras well known for their chronicle of life: the oldest Paleozoic, middle Mesozoic, and current Cenozoic. The Proterozoic (“before com- plex life”) Eon precedes the Phanerozoic, extending back 2.5 billion years. The Archean (“ancient”) and Hadean (“unseen”) Eons reach back to the formation of the Earth. The Precambrian Supereon combines the oldest three eons, and refers to the time before the first great explosion of life recorded in the fossil record - the Cambrian Period. The name “Cambrian” refers to Wales, where these fossils were first studied. Before this first period of the Phanerozoic, animals lacked hard body parts to contribute to the fossil record.
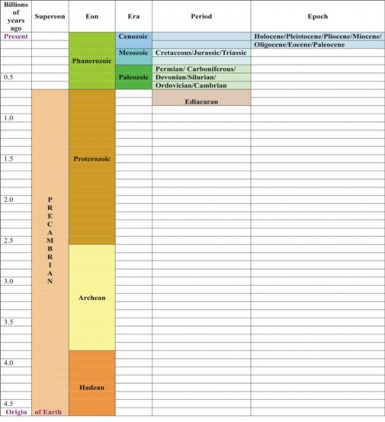
www.ck12.org 490
Throughout geologic time, the fossil record reveals dramatic changes in species and groups of species which have populated the Earth. Evolution at or above the species level is macroevolution, in contrast to microevolution, which describes changes within a species or population. Many scientists no longer emphasize the distinction, believing evolution to be a single process which includes both patterns. However, themes from the Geologic Time Scale illustrate macroevolution, so we will consider its patterns and processes in this chapter.
Although fossils dated back only to the Cambrian during Darwin’s time, radiometric dating has since identified fossil bacteria as old as the beginning of the Archean era 3.5 billion years ago. The geologic record shows over 2 billion years during which the only life was unicellular. The appearance of eukarytoic cells roughly 1.8 billion years ago marked a dramatic increase in cellular complexity. In rocks 1 billion years old, multicellular eukaryotes begin to appear, and by the end of the Precambrian, fossils record a variety of ancient multicellular organisms. The beginning of Cambrian Period marks an “explosion” of life, and in general, biodiversity has increased throughout the Phanerozoic, shown in Figure 11.9. Our current understanding of the fossil record confirms Darwin’s ideas that life began as tiny single-celled bacteria and over vast time evolved to produce the complexity and diversity we celebrate today.
As recorded in fossils, the evolution of life was not smooth or steady. Mass extinctions and episodic speciation interrupted the overall pattern of increasing biodiversity, shown in Figure 11.9. These disruptions reflect dramatic changes in the environment of the Earth.
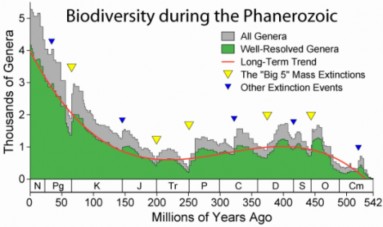
A major theme of the fossil record is loss of species. The death of a species – extinction
seems to be as much a characteristic of life as the death of individual organisms. Both
seem closely linked to change in environment. Through mutation or sexual reproduction, offspring show variation. Individuals whose variations are not well suited to their environ- ment die. Those whose variations are adaptive survive to reproduce. Death and differential reproduction result in adaptation to a changing environment.
These same forces of natural selection inevitably affect species: the fossil record indi- cates that up to 99.9% of all species that have ever lived on Earth are now extinct. Mass extinctions involve most major groups of organisms over a short period. The past 550 million years, when fossils are sufficiently abundant to tell a reliable story, show five mass extinctions in which more than 50% of animal species died. The most famous is the ex- tinction which ended the reign of the dinosaurs 65 million years ago. Accelerated evolution may follow mass extinction, because the empty ecological niches make way for new species. After the non-birdlike dinosaurs disappeared, mammals rapidly evolved to fill the available niches. The fossil record shows numerous examples of episodic speciation, a pattern of periodic increase which includes these rebounds as well as bursts of evolution following major new “discoveries” or “ideas” – for example, the biochemical pathways for photosynthesis or cellular respiration.
Closely related to mass extinction is the theme of major environmental change throughout Earth’s history. Rock layers reflect critical changes in atmosphere and climate: oxidized iron deposits mark the introduction of oxygen gas to the atmosphere, and glacial deposits reflect periodic ice ages alternating with times of global warming. Craters and unique world- wide strata suggest that spectacular asteroid or comet collisions may have severely reduced solar radiation, and lava flows and ash suggests volcanism could have done the same. Mas- sive geographic changes, now explained by plate tectonics theory, underlie volcanism as well as formation of new land bridges, seaways, and continents. Certain worldwide sedi- mentary deposits suggest significant sea level fluctuation, which may result from some of the aforementioned climate or plate tectonic changes. Life evolved against the backdrop of these often-catastrophic changes, and over 3.5 billion years of natural selection inevitably responded to them. Many of these changes are believed to have caused the mass extinctions and episodic speciation revealed in the fossil record. We will look at some of these events in more detail in the next two lessons.
Two caveats are critical in interpreting the history of life using the Geologic Time Scale. The first concerns the idea that evolution progresses via slow, steady, gradual change. We have already seen that mass extinction and episodic speciation interrupt the overall pattern of increasing biodiversity, but gradualism suggests that changes accumulate continuously as one species evolves to become another. An alternative, more recent theory, punctuated equilibrium, shown in Figure 11.10, proposes that species remain the same for long periods, and that change occurs infrequently but rather rapidly under unusual conditions such as geographic isolation or migration. The rather sudden appearance and disappearance of many individual species within the fossil record, noted even by Darwin, tends to support the latter theory. The idea of quantum evolution attempts to explain the origins of major groups (families, orders, and classes) as a response to drastic changes in environment or
www.ck12.org 492
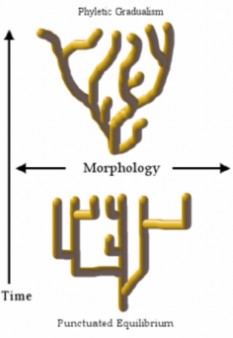
Figure 11.10: Two theories of evolutionary change - gradualism vs. punctuated equilibrium
adaptive zones. The fossil record supports great variation in the rate of evolutionary change
from group to group and even among closely related lineages. Each of these ideas about pattern and rate may accurately describe one of many ways evolution works.
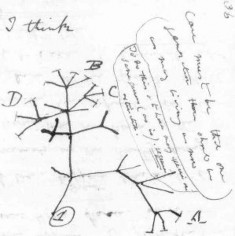
A second caveat: the 4.6 billion year time scale makes it tempting to view evolution as linear, and perhaps even goal-directed. Time may be an arrow, but evolution is much more a bush of common ancestry–a family tree, as we saw at the beginning of the chapter. Darwin recognized this - his sketch, shown in Figure 11.11, shows the pattern of speciation predicted by his theory of chance variation and adaptive selection. A very recent (August 2007) discovery encourages us to view our own human ancestry as a bush rather than a line. Radiometric dating of a new fossil of Homo habilis shows that this species coexisted with the ”more advanced” Homo erectus, shown in Figure 11.12. Previously, scientists considered the former an ancestor of the latter. The inappropriate expression “more advanced” implies the false, linear, goal-directed interpretation of evolution.
A famous example of “bushiness” in the history of life is adaptive radiation, a type of divergent evolution. This pattern of speciation involves the relatively rapid evolution from a single species to several species which fill a diversity of available ecological niches. Mass extinctions (the dinosaurs!), new volcanic islands (the Galapagos, or Hawaii), land bridge formation (the isthmus between North and South America) or “invention” of a new idea in evolution – all are events which “suddenly” open a variety of niches for adaptive radiation. In each case, a fundamental structure in one species is modified to serve new functions in different environments or modes of life. For example, forelimbs of mammals have become elongated with grasping hands for the forested habitats of monkeys, flattened into flippers for the aquatic habitats of whales, and spread into wings for the aerial habitats of bats, shown in Figure 11.13. Adaptive radiation explains – and the fossil record shows – that these groups all arose from one ancestor or a small group of common ancestors.
www.ck12.org 494
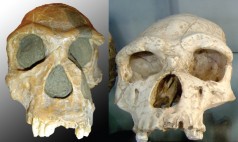
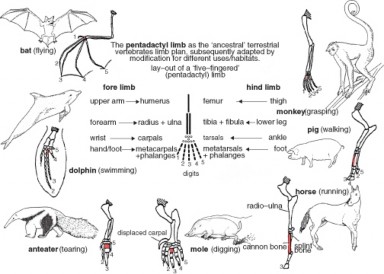
In contrast to divergent evolution, whereby closely related species evolve different traits, con- vergent evolution involves distantly related species evolving similar traits. This pattern surfaces frequently in the history of life when different organisms occupy similar ecological niches. For example, three major groups of organisms have evolved wings for flight: reptiles (pterosaurs), birds, and mammals (bats), shown in Figure 11.14.
Australian fauna reveal both divergent and convergent patterns related to major geographical change, shown in Figure 11.15. Major groups of mammals evolved in the northern hemi- sphere and migrated to Australia across a land bridge. Later submerging of the land bridge isolated the Australian mammals, and the marsupials underwent their own adaptive radia- tion within their insular continent. Elsewhere, placental mammals evolved to out-compete the more primitive monotremes and marsupials, and underwent their own adaptive radiation. These independent radiations resulted in some wonderful examples of convergent evolution: An example: the marsupial Tasmanian wolf (now extinct) shared with the placental canines many adaptations to life as a hunting predator, shown in Figure 11.16.
One last, fascinating pattern within the history of life is coevolution. In coevolution, two species or groups of species influence each other’s evolution and therefore evolve in tandem. Relationships may be positive for one species or both, or an evolutionary arms race between predator and prey. Flowering plants depend on insects for pollination, so have evolved colors, shapes, scents, and even food supplies which are attractive to certain
www.ck12.org 496

insect species. Insects, in turn, have evolved mouthparts, senses, and flight patterns which allow them to respond to and benefit from specific floral “offerings,” shown in Figure 11.17. The Endosymbiotic Theory describes a special form of co-evolution: Mitochondria and chloroplasts evolve within eukaryote cells, yet because these organelles have their own DNA sequence, different from that of the nucleus in the “host” cell, organelle and host cell evolve in tandem – each influences the evolution of the other.

Closely related to coevolution is coextinction. If one member of a pair of interdependent species becomes extinct, the other is likely to follow. Famous examples were two species of bird lice which were obligate parasites on the passenger pigeon, shown in Figure 11.18.
www.ck12.org 498
When “Martha,” a resident at the Cincinnati Zoo thought to be the last passenger pigeon in the world died on September 1, 1914, the extinction of the bird lice species followed. Alas, one louse species was later rediscovered on a band-tailed pigeon, and the other species had been misidentified.

In this lesson, you have explored the tools we use to study the history of life, the Geologic Time Scale which organizes what we know, and a variety of patterns found in the 4.6 billion year story. In the next lessons, look for examples of these patterns as we follow that story from the origin of life to what is becoming known as the Sixth Extinction today.
If the history of life is condensed into a single 24-hour “cosmological day,” humans occupy only the last minute and one-half, and civilization, less than the final second.
Fossils include mineralized remains of organisms, casts, impressions, footprints, bur- rows, droppings, eggs, or nests as well as frozen, dried, or amber-coated remains.
The positions of fossils in rocks indicate their relative ages; older fossils and rock layers are deeper than fossils and rocks that are more recent.
Radiometric dating measures the proportion of decay products of radioisotopes with known half-lives to estimate absolute age. Different isotopes with a range of half-lives cover the span of geologic time.
Comparing DNA or protein in similar species can reveal evolutionary relationships and confirm patterns suggested by the fossil record.
Geologic Time Scale divisions mark major events which highlight changes in climate, geography, atmosphere, and life.
The largest units of time are Eons; the 4.6 billion years of earth’s history are divided into four eons.
The Phanerozoic includes the most recent 545 million years and the most detailed fossil record.
Overall, the fossil record confirms Darwin’s idea that life began as tiny single-celled bacteria and over time evolved to produce the complexity and diversity we celebrate today.
Mass extinctions and episodic speciation interrupt an overall gradual increase in com- plexity and diversity.
Evolution shows response to major environmental changes, including volcanism, con- tinental drift, sustained warming and cooling, asteroid impact, and critical transfor- mations of earth’s atmosphere.
The rate of evolution is not always uniform; gradualism does not characterize all spe- ciation.
The pattern of evolution is seldom linear, but rather more like a bush or family tree.
Punctuated equilibrium, quantum evolution, and variable rates describe patterns of evolution which may differ from gradualism.
In divergent evolution, also called adaptive radiation, closely related species evolve different traits to adapt to a variety of available niches.
In convergent evolution, distantly related species evolve similar traits as adaptations to similar habitats.
Geographic changes, including continental drift, affect patterns of evolution.
How do the conditions needed for fossilization explain the rarity of fossils?
Compare relative dating of fossils and rock layers to absolute dating.
Explain why “carbon dating” is an inadequate description of aging rocks and fossils.
Describe how molecular clocks clarify evolutionary relationships.
Compare and contrast Geologic Time with absolute time, including the limits of each.
Explain the ways in which the Geologic Time Scale and the fossils it records may be misleading concerning the history of life.
Construct a table or chart which shows five of the major patterns of macroevolution we have observed in the fossil record. Include the pattern name, a brief description or definition, causes or contributing factors (where applicable), and a specific example for each.
Explain how some of the patterns of evolution and environmental change account for worldwide differences in the distribution of mammals. Discuss placentals vs. marsupi- als.
Charles Darwin described an orchid from Madagascar that had a nectar well which measured 12 inches deep, keeping costly sugars far out of reach of all known butterflies and moths. He predicted the existence of a highly specialized pollinator moth with
www.ck12.org 500
a foot-long proboscis that could act as a straw to reach the nectar. After Darwin’s death, scientists discovered a night-flying moth that matched Darwin’s expectation and named it the “Predicta.” Which pattern of evolution did Darwin see in the orchid? Explain why this is a good example of the pattern.
Relate human history to life’s history, as shown in the Geologic Time Scale and fossil record.
Colleen Whitney, Kate Barton, David Smith, “The Paleontology Portal.” University of California Museum of Paleontology, Paleontological Society, Society of Vertebrate Paleontology, and US Geological Survey, 2003. Available on the web at:
“Continental Drift Animation.” EduMedia-sciences, 2002-2007. Available on the web at
http://www.edumedia-sciences.com/a95_l2-continental-drift.html
Dave Smith, “Life Has a History – Level 2.” University of California Museum of Pale- ontology, 7/18/06. Available on the web at:
http://www.ucmp.berkeley.edu/education/explorations/tours/intro/Intro5to12/ tour1nav.php
Jim Kurpius. Rob Guralnick, Jennifer Johnson, Anne Monk,Judy Scotchmoor, and
Mark Stefanski, “Understanding Geologic Time.” University of California Museum of Paleontology, 1994-2007. Available on the web at:
http://www.ucmp.berkeley.edu/education/explorations/tours/geotime/index. html
Lexi Krock, “The Missing Link: A Brief History of Life.” Nova Online, last updated
February 2002. Available on the web at:
Roy Caldwell and David R. Lindberg, “Evolution 101.” University of California Mu- seum of Paleontology, 2007. Available on the web at:
http://evolution.berkeley.edu/evolibrary/article/evo_01
absolute aging Measures half-lives of radioactive isotopes to calculate the number of years which have elapsed since a rock formed; also known as absolute dating.
adaptive radiation A pattern of speciation which involves the relatively rapid evolution from a single species to several species to fill a diversity of available ecological niches.
coevolution Evolution in which two species or groups of species influence each other’s evolution and therefore evolve in tandem.
coextinction If one member of a pair of interdependent species becomes extinct, the other is likely to become extinct as well.
convergent evolution Evolution whereby distantly related species evolving similar traits. divergent evolution Evolution whereby closely related species evolve different traits. eons The largest units of time within the Geologic Time Scale; divided into eras, which
are also divided into periods, epochs, and stages.
episodic speciation A pattern of periodic increase in new species; follows mass extinctions as well following major new “discoveries” or “ideas” – for example, the biochemical pathways for photosynthesis or cellular respiration.
extinction The death of an entire species.
fossils The preserved remains or traces of organisms; provide extremely rare but vivid windows to the past.
Geologic Time Scale A column of rock layers which reflects the history of sedimentary rock formation and changing life.
gradualism The idea that evolution progresses via slow, steady, gradual change; suggests that changes accumulate continuously as one species evolves to become another.
index fossils Widespread, short lived fossils that can be used to help identify rock layers of the same age spread around the Earth, providing the relative age of other fossils.
macroevolution Evolution at or above the species level.
microevolution Describes changes within a species or population.
molecular clocks Measure changes in DNA or proteins to indicate degrees of relationship among species.
paleontologists Scientists who study fossils.
punctuated equilibrium Proposes that species remain the same for long periods, and that change occurs infrequently but rather rapidly under unusual conditions such as geographic isolation or migration.
www.ck12.org 502
quantum evolution Proposes that the origins of major groups (families, orders, and classes) occurred as a response to drastic changes in environment or adaptive zones.
trace fossils Fossils consisting of footprints, burrows, droppings, eggs, nests, and other types of impressions.
Consider the range of tools used to study a history which no human could witness. These range from fossils – the actual remnants of living organisms – to comparisons of molecules within organisms still living today. Which tools do you consider most reliable? Does the fact that the information from one set of tools often confirms that evidence collected using a different set of tools strengthen your acceptance of the data?
Review the various patterns of macroevolution, from mass extinction to coevolution and coextinction. Which of these best support the depiction of evolution as a bush, rather than an arrow? Which support the idea that evolution builds on what already exists, so the more variety there is, the more there can be in the future?
Relate the nature of science to our current understanding of the origin of life.
Describe the formation of the atoms which build the Earth and its life.
Explain the formation of the moon, and its effects on Earth’s conditions for life.
Compare and contrast Earth’s early atmosphere with today’s atmosphere.
Discuss the formation of Earth’s early atmosphere and oceans.
Indicate the age of the Earth and identify supporting evidence.
Interpret the importance of Miller and Urey’s experiment.
Relate the properties of phospholipids to the formation of the first membranes.
Compare and contrast the “genes-first” model of the origin of life to the “metabolism first” model.
Explain why some scientists believe that RNA was the basis of early life.
Evaluate the hypothesis that exogenesis explains the origin of life on Earth.
Describe the theoretical characteristics of the first cell.
Discuss the concept of a “LUCA,” or last universal common ancestor.
Indicate the origin of photosynthesis and its consequences for Earth’s life and atmo- sphere.
Analyze the effects of the development of atmospheric oxygen on life.
Explain the importance of the emergence of cellular respiration.
Explain the Endosymbiotic Theory of the origin of eukaryotic cells.
Evaluate the evidence for the Endosymbiotic Theory.
Identify the origins of the three major domains of life.
Analyze the evolutionary potential of the eukaryotic cell.
Discuss the pros and cons of the evolutionary “tree” as a way of depicting the evolu- tionary process.
No part of the story of life holds more mystery or fascination than its ultimate origins. Cos- mologists, geologists, paleontologists, and biologists have collected, compared, scrutinized, evaluated, and revised many kinds of evidence in order to see into the past. As a result, well- accepted theories now illuminate nearly 4 billion years of life’s history, 4.6 billion years of Earth’s history, and even 13.7 billion years back to the Big Bang, which began the universe as we know it. Yet until the 19th century, most people believed the Earth was just 6,000 years old. We still do not know whether life exists beyond our Earth, nor can we predict where evolution will take life on Earth in the future, and our theories leave many chapters of the story untold. As you explore the early history of life, you must remember that the nature of science is to continue to question its own conclusions, to persist in seeking new information, and to readily modify or even overturn long-accepted theories, if new evidence contradicts them. This lesson includes some of the best explanations science can currently provide for life’s origin and early evolution. A story of stardust, explosions, collisions, com- petition, and cooperation should not disappoint you, but it probably won’t give you all the answers you seek. If this lesson provides insight and raises more questions, you will have a firm foundation upon which to build future understanding as it unfolds. Perhaps you could join the search!
We will begin our story of the origin of life by exploring the origin of the materials which build it. The materials have a beauty and diversity of their own; perhaps your study of the Periodic Table of the Elements gave you an appreciation for their variety and individuality. Earth began as the solar system began – often described as a giant rotating cloud of dust, rocks, and gas. “Dust, rocks, and gas” may not sound inspiring, but this cloud contained the 92 elements or kinds of atoms which somehow combine to form every corner – living and nonliving - of the exquisite world we inhabit. The Big Bang (9 billion years earlier!) produced the atoms of hydrogen and helium. Elements as heavy as lithium followed the Big Bang within minutes. Stars such as red giants fused hydrogen and helium nuclei to form elements from carbon (the foundation of life!) to calcium (now our bones and teeth). Supernova explosions formed and ejected heavier elements such as iron (for red blood cells). We are not just “dust.” We - and our world - are stardust!
www.ck12.org 504
How did this rotating cloud of stardust become our solar system? One theory suggests that a nearby supernova sent a shock wave through the cloud, increasing its spin to form a protoplanetary disk, shown in Figure 11.19. Most of the mass concentrated in the middle and began to heat up, but large debris and collisions resulted in concentrations of matter outside the center. Eventually, heat in the central core began nuclear fusion of hydrogen to helium, and the Sun ignited. Matter outside the Sun’s gravity separated into rings of debris, and collisions of objects within the rings formed larger objects, which eventually became the planets. Solar wind cleared much of the remaining non-planetary material from the disk.

One of the collections of debris, approximately 150 million kilometers from the Sun, was the protoplanet Earth. Newborn, Earth was very different from the home we know today. Bombarded by debris and heated by radioactive decay and the pressure of contraction, the Earth at first was molten. Heavy elements sank to the center, and lighter ones surfaced. Heat and solar wind meant that no atmosphere and no oceans were present.
Eventually, contraction and cooling allowed formation of a crust and retention of an atmo- sphere. However, continued bombardment melted portions of the crust for long periods. About 4.5 billion years ago, Earth collided with another protoplanet, Theia. This “big whack” gave us our moon and tilted Earth on its current axis, leading to the seasons, which now influence so much of life’s diversity. The Big Whack may also have initiated plate tec- tonic activity by speeding up the Earth’s rotation. Since then, however, the moon’s tidal drag may be slowing that rotation; scientists suggest that the day/night cycle during the Hadean may have been as short as 10 hours.
As the Earth continued to cool amidst heavy bombardment, steam escaped from the crust and active volcanoes released other gases to form a primitive atmosphere, which contained ammonia, methane, water vapor, carbon dioxide, and nitrogen, but no more than a trace of oxygen. In the absence of oxygen, no ozone layer protected Earth from the Sun’s ultraviolet rays. Between 4.2 and 3.8 billion years ago, clouds and rain formed the oceans. The oceans were olive green, and the reddish atmosphere would have been toxic to modern multicellular
organisms. Yet the stage was set for life to begin.
The Hadean Eon ended 3.8 billion years ago, its timeline marked by Earth’s oldest known rocks (between 3.8 and 4.2 billion years old) and oldest known minerals (formed 4.4 billion years ago). Scientists use these dates to estimate that the Earth itself is 4.6 billion years old. Evidence for life during the Hadean does not exist, although many scientists push the theoretical origin back that far. How – and when – did life arise?
Once again, we will begin with the materials of life – this time, organic molecules, made primarily of the element carbon. Most scientists agree that these organic molecules arose before cells, which we now consider essential to the definition of life. Several hypotheses and experiments suggest ways in which organic building blocks may have formed.
In 1924, Aleksandr Oparin proposed that life could have developed through gradual chemical evolution in a “primordial soup.” In 1953, Stanley Miller and Harold Urey designed a now-famous test of the hypothesis that the conditions of primitive Earth favored chemical reactions that synthesized organic molecules from inorganic precursors. Their experiment (Figure 11.20) showed that a mixture of gases, believed to be part of the primitive Earth atmosphere, when subjected to sparks representing lightning, formed a mixture of monomers representing each of the four major groups of organic molecules. Although DNA, RNA, and polymers were absent, 13 of the 22 amino acids that make up modern protein, plus lipids, sugars, and some building blocks of DNA and RNA, were among the products of the experiment.
The “leap” from building blocks to polymers and from organic soup to individual replicating units has been more difficult to demonstrate. In the ‘50s and ‘60s, Sydney Fox showed that early Earth conditions could result in short chains of amino acids, which in turn could form enclosed spheres. Phospholipids can self-organize into membranes in a similar fashion, and cell membranes today consist primarily of a bi-layer of these lipids. Phospholipids or polypeptides could have surrounded and protected early metabolic units, forming protocells shown in Figure 11.21, simple membrane-enclosed spaces which may have led to the later evolution of true cells.
Walter Gilbert, Carl Woese, and Alexander Rich proposed that RNA, because it can serve both catalytic and replicating functions, was the first informational molecule, and formed the “RNA World Hypothesis” for the origin of life. Sol Spiegelman created a short chain of RNA which was able to replicate itself in the presence of RNA polymerase; the segment is now known as the “Spiegelman monster.” The idea that a successful replicator molecule preceded the evolution of biochemical pathways is the “Genes-First” model.
In contrast, Günter Wächtershäuser proposed that sulfides of iron and other minerals con- www.ck12.org 506
tain energy which could have polymerized basic building blocks. He argued that extensive evolution of biochemical pathways might have preceded replicator molecules and individu- alization of life. His ideas formed the basis of William Martin and Michael Russell’s 2002 hypothesis that black smokers at seafloor spreading zones, shown in Figure 11.22, could have provided conditions for extensive chemical and biochemical pathway evolution. Their reasoning suggests that lipid membranes allowing independent lives away from the smokers could have been a last step in early evolution. The fact that archaebacteria and eubacte- ria (and us eukaryotes!) have completely different membrane lipids but similar metabolism supports the concept of early biochemical pathway evolution. These ideas comprise the “Metabolism-First” model.
The discovery of organic molecules in space supports the exogenesis hypotheses which propose that life could have originated elsewhere – on Mars, or at some distant point in the universe. Comets and meteorites are known to contain organic molecules, and could have delivered them to Earth. Exogenesis does not really answer the question of how life originated, but provides a much wider temporal and spatial framework in which it could have happened.
www.ck12.org 508
Although many hypotheses and some experiments and observations explore the origin of cellular life, actual events remain unknown. If earth’s life first arose on earth, rather than by exogenesis, its timing is speculative, for no fossils record that event. Admitting that many conflicting hypotheses exist, we often express our current understanding of the story this way:
Perhaps four billion years ago during the Hadean Eon, lightning and a primitive atmosphere produced an organic soup of chemicals. As the “soup” became more concentrated, molecules began to interact with one another. As molecules became more complex, some molecules helped to speed up or catalyze chemical reactions (perhaps RNA, but eventually protein). Within that highly reactive soup, a molecule gained the ability to copy itself, becoming the first replicator (perhaps RNA, but eventually DNA). Copies contained errors, and errors which prevented replication caused the copies to “die out.” Copies that replicated faster sur- vived to make more copies. Eventually, lipid membranes surrounded some of these chemicals, protecting them from reacting with other chemicals.
Although many protocell “species” probably populated the early “soup,” scientists believe that only one – a last universal common ancestor (LUCA) – emerged about 3.5 billion years ago during the Archean Eon, and later gave rise to all cellular life on earth. This prokaryote probably had a cell membrane and ribosomes, and used DNA for information storage, RNA for information transfer, and protein for catalyzing chemical reactions – like all life today. The first cells were probably heterotrophs, feeding on energy-rich chemicals concentrated in the “soup.” Alternatively, they could have been chemoautotrophs, extracting energy from inorganic molecules. Not long after prokaryotic cells emerged, they split into two major groups, Eubacteria and Archaebacteria. Both persist today, although Archaebacteria more often inhabit extreme habitats.
Inevitably, a diminishing supply of food molecules led to competition. At some point, gly- colysis evolved as a pathway for transferring energy from organic molecules to ATP. This pathway persists in almost all organisms today.
Eventually, about three billion years ago, a new strategy evolved among some prokaryotes, which used sunlight to make carbohydrates from carbon dioxide and water. Photosyn- thesis provided a new source of food molecules for both autotrophs and the heterotrophs that “learned” to consume them. The oldest fossils, stromatolites, (Figure 11.23) record abundant photosynthetic cyanobacteria from that time.
Oxygen produced by photosynthesis first oxidized iron dissolved in the oceans, creating massive deposits of iron ore. Eventually, toward the end of the Archean, oxygen began to accumulate in the atmosphere, creating a major environmental change that is sometimes called the “Oxygen Catastrophe.” Oxygen was indeed toxic to many of the prokaryotes which had evolved as anaerobes. However, ultraviolet rays converted some of the oxygen to ozone, which prevented much of that harmful radiation from reaching the earth’s surface.

Thus, while an oxygen atmosphere may have killed many species, it allowed survivors to colonize previously uninhabitable ocean surface and terrestrial habitats. Even more impor- tant to the future of life, some prokaryote survivors “learned” how to use oxygen to harvest a great deal more energy from organic molecules. The energy efficiency of aerobic res- piration paved the way for the emergence of larger and more complex organisms in the Proterozoic Eon.
You have learned that our own eukaryotic cells protect DNA in chromosomes with a nuclear membrane, make ATP with mitochondria, move with flagella (in the case of sperm cells), and feed on cells which make our food with chloroplasts. All multicellular organisms and the unicellular Protists share this cellular intricacy. Bacterial (prokaryotic) cells are orders of magnitude smaller and have none of this complexity. What quantum leap in evolution created this vast chasm of difference?
The widely accepted Endosymbiotic Theory, shown in Figure 11.24, proposes that many organelles were once independently living cells. Larger cells engulfed these smaller cells but did not digest them, perhaps due to prey defenses. Alternatively, perhaps the smaller cells invaded the larger cells with the “intent” to parasitize. In either case, with their own DNA, the endosymbionts reproduced independently within the cell, and cell division passed them on to future generations of cells. Aerobic bacterial invaders would have been able to use oxygen to further break down and use energy from the host’s “wastes” from glycolysis. So much energy (ATP) resulted that some was available to the host; a mutually beneficial symbiosis resulted. This intriguing story of cooperation – so different from natural selection’s emphasis on competition – explains the origin of our mitochondria. A similar tale is told for chloroplasts; the benefit for a heterotrophic “host” is clear. Some scientists view cilia, flagella, peroxisomes, and even the cell nucleus as endosymbionts, but these ideas are less widely accepted.
www.ck12.org 510
What is the evidence for this maverick evolutionary pathway? Biochemistry and electron microscopy provide convincing support for the Endosymbiotic Theory. The mitochondria and chloroplasts which live within our eukaryotic cells share the following features with prokaryotic cells:
Organelle DNA is short and circular – and sequences do not match DNA in the nucleus.
Molecules that make up organelle membranes resemble those in prokaryotic membranes
– and differ from those in eukaryotic membranes.
Ribosomes in these organelles are similar to those of bacteria – and different from eukaryotic ribosomes.
Reproduction is by binary fission – not mitosis.
Biochemical pathways and structure show closer relationships to prokaryotes.
Two or more membranes surround these organelles.
The “host” cell membrane and biochemistry are more similar to those of Archaebacteria, so scientists believe eukaryotes descended more directly from that major group (Figure 11.25). However, the standard evolutionary tree cannot accurately depict our ancestry, because the origin of the eukaryotes combines traditional descent from the Archaea with landmark cohabitation alliances forged with the Bacteria.
The timing of this dramatic evolutionary event (more likely a series of events) is not clear. The oldest fossil clearly related to modern eukaryotes is a red alga dating back to 1.2 billion years ago. However, many scientists place the appearance of eukaryotic cells at about 2 billion years. Some time within Proterozoic Eon, then, all three major groups of life – Bacteria, Archaea, and Eukaryotes – became well established. Geologists hypothesize the oldest supercontinent, Columbia, between 1.8 and 1.5 years ago, as the backdrop for the further evolution of these three domains.
Eukaryotic cells, made possible by endosymbiosis, were powerful and efficient. That power and efficiency gave them the potential to evolve new ideas: multicellularity, cell special- ization, and large size. They were the key to the spectacular diversity of animals, plants, and fungi which populate our world today. We will tell their much more familiar story in the next lesson. Nevertheless, as we close the history of early life, reflect once more on the remarkable but often unsung patterns and processes of early evolution. Our “size-ism” sets us up to wonder at plants and animals, and ignore bacteria. Our human senses cannot di- rectly perceive the unimaginable variety of single cells, the architecture of organic molecules, or the intricacy of biochemical pathways. Let your study of early evolution give you a new perspective – a window into the beauty and diversity of unseen worlds – now and throughout Earth’s history. Apart from the innumerable mitochondria which call your 100 trillion cells home, your body contains more bacterial cells than human cells. You, mitochondria, and your resident bacteria share common ancestry – a continuous history of the gift of life.
www.ck12.org 512
The Big Bang and stars such as red giants made the atoms which build life.
Earth gradually condensed into a molten protoplanet, constantly bombarded with de- bris.
Steam escaping from the formative crust and volcanic gases contributed to Earth’s early atmosphere, which probably contained methane, ammonia, carbon dioxide, water and nitrogen. Such an atmosphere would be toxic to most modern organisms.
No oxygen meant no ozone; ultraviolet radiation reached the Earth and threatened life with deadly, mutating rays.
Eventually, water in the atmosphere condensed into clouds and rain, forming oceans.
Earth’s oldest known rocks are between 3.8 and 4.2 billion years old. The oldest minerals are 4.4 billion years old. Scientists estimate that the age of the Earth is 4.6 billion years.
Miller and Urey showed that a spark igniting a mixture of gases resembling Earth’s primitive atmosphere could produce most of the building block organic molecules of life – forming an “organic soup.”
Some lipids and certain polypeptides can spontaneously form into protocells; early membranes could have self-organized in this way.
The “Genes-first” hypothesis proposes that replicating molecules evolved before bio- chemical pathways.
Some scientists believe RNA, rather than DNA, was the first replicator.
The “metabolism-first” model suggests that biochemical pathways evolved in an or-
ganic soup before self-replicating molecules.
Many scientists accept that a “last universal common ancestor” (LUCA) cell arose from the primeval soup of organic molecules.
This prokaryote probably had a cell membrane and ribosomes, and used DNA for information storage, RNA for information transfer, and protein for catalyzing chemical reactions – like all life today.
The first cells were probably heterotrophs feeding on organic soup, or chemoautotrophs using the energy in inorganic molecules.
Not long after the LUCA prokaryote arose, life split into two groups, Bacteria and the Archaebacteria.
Photosynthesis arose roughly 3 billion years ago.
The oldest fossils, stromatolites, preserve photosynthetic cyanobacteria.
Oxygen produced by photosynthesis eventually changed Earth’s atmosphere.
Ozone formed, protecting life from damaging UV radiation.
The widely accepted Endosymbiotic Theory explains the origin of eukaryotic cells as a merging of several kinds of prokaryotic cells.
Why is understanding the nature of science important to studying the origin of life on Earth?
Interpret the statement “we are made of stardust.”
Describe the effects of the moon on the conditions for life on Earth, according to the impact theory of the moon’s origin.
Discuss the formation of Earth’s atmosphere and compare it to today’s.
Identify the age of the Earth, and give the supporting evidence.
Describe Miller and Urey’s experiment, and evaluate its importance to our understand- ing of the origin of life.
Compare and contrast the RNA World, genes first, metabolism first, and exogenesis models of the origin of life. Evaluate the evidence supporting each model.
List the characteristics scientists attribute to “last universal common ancestor” of life on Earth.
Indicate when scientists believe photosynthesis originated, and what evidence suggests this. Analyze the effects of the origin of photosynthesis on life existing at that time.
Analyze the theory which explains our current understanding of the origin of eukaryotic cells. In what way does it differ significantly from “traditional” ideas of evolution?
Mark Pagel, ed. The Oxford Encyclopedia of Evolution. New York: Oxford University Press, 2002.
www.ck12.org 514
Stephen Jay Gould, ed. The Book of Life: An Illustrated History of the Evolution of Life on Earth. New York: W.W. Norton, 1993.
Lynn Margulis et al. Five Kingdoms: An Illustrated Guide to the Phyla of Life on Earth. New York: W.H. Freeman, 1998.
Cain et al., “Endosymbiosis Animated.” From Discover Biology, Third Edition, W. W. Norton & Co.© 2006 W. W. Norton & Co. and Sumanas, Inc.Available on the Web at:
http://www.sumanasinc.com/webcontent/anisamples/nonmajorsbiology/organelles. html
Dave Smith, “Life Has a History – Level 2.” University of California Museum of Pale-
ontology, 7/18/06. Available on the Web at:
http://www.ucmp.berkeley.edu/education/explorations/tours/intro/Intro5to12/ tour1nav.php
Lexi Krock, “The Missing Link: A Brief History of Life.” Nova Online, last updated
February 2002. Available on the Web at:
Richard Cowen, “History of Life, 4th edition Updates, References and weblinks.” UC Davis Geology Department, 3 March 2006. Available on the Web at:
Roger Perkins, “The Virtual Fossil Museum: Fossils across Geologic Time and Evolu- tion.” Available on the web at:
Roy Caldwell and David R. Lindberg, “Evolution 101.” University of California Mu- seum of Paleontology, 2007. Available on the Web at:
Roy Caldwell and David Lindberg, “Understanding Evolution.” University of California Museum of Paleontology, 2007. Available on the Web at:
http://evolution.berkeley.edu/
endosymbiotic theory Theory which proposes that many organelles were once indepen- dently living cells; describes the formation of eukaryotic cells.
genes-first model The idea that a successful replicator molecule preceded the evolution of biochemical pathways.
LUCA The last universal common ancestor; the first true cell, which formed about 3.5 billion years ago.
metabolism-first model The proposal that extensive evolution of biochemical pathways might have preceded replicator molecules and individualization of life.
organic molecules The “materials of life” - molecules made primarily of the element car- bon.
oxygen catastrophe Toward the end of the Archean, oxygen began to accumulate in the atmosphere, killing many anaerobic species.
primeval soup Oceans in which gradual chemical evolution formed life; proposed by Alek- sandr Oparin.
protocells Simple, membrane enclosed early metabolic units surrounded by phospholipids or polypeptides; precursors to true cells.
RNA world hypothesis Hypothesis that proposes that RNA evolved prior to DNA.
Which theory of life’s origins do you consider most plausible: genes first, metabolism first, or exogenesis? What kinds of evidence would be required to support each theory?
The standard form for an evolutionary tree is a series of branching lines which show common ancestors. Can you imagine a format which could show Endosymbiosis, as well as common ancestry?
Assess the impact of global environmental changes on the evolution of life.
Describe the diversity of unicellular organisms which arose over 2 billion years of evo- lution.
Evaluate the importance of major evolutionary developments which preceded the Cam- brian explosion: colony formation, cell specialization, and sexual reproduction.
Evaluate the importance of some factors which contributed to the “Cambrian explo- sion” of biodiversity.
Trace the evolution of plants and animals from aquatic to terrestrial habitats.
Connect changes in atmospheric O2 and CO2, temperature, geography, and sea level to extinctions and radiations of various groups throughout the Paleozoic.
Identify recurrent extinctions as losses of diversity, but also opportunities for the evo- lution of new species.
Describe the conditions under which the dinosaurs emerged to dominate life on Earth. www.ck12.org 516
Identify the diversity of habitats and niches occupied by the dinosaurs during their “golden age.”
Discuss the relationships between reptiles, birds and mammals during the age of the dinosaurs.
Explain the coevolution of flowering plants and insects during the Cretaceous.
Evaluate the evidence for an “impact event” as the primary cause of the K-T extinction which ended the reign of the dinosaurs.
Analyze the emergence of mammals and birds as the dominant land animals during the early years of the Cenozoic.
Connect sea level, land bridges, and climate to their effects on evolution.
Explain the connection between CO2 levels, temperature, and glaciation.
Discuss the factors which contribute to the “sixth” major extinction.
Biologists estimate that 99% of the species which have ever lived on Earth are now extinct, and up to 80 million species populate our world today. It is the great diversity of species that allows at least some organisms to survive major changes in the environment.
4 billion years of simple, prokaryotic cells
3 billion years of photosynthesis
2 billion years of complex, eukaryotic (but still single!) cells
1 billion years of multicellular life
The history of life reaches the last billion years of Earth’s 4.6 billion-year history with no hint of the wondrous diversity of life as humans know it. Not until nearly 80% of Earth’s history had passed did multicellular life evolve. The fossil record tells the story: millions of species of fish, amphibians, reptiles, birds, mammals, mosses, ferns, conifers, flowering plants, and fungi populated the seas and covered the Earth - as continents crashed together and broke apart, glaciers advanced and retreated, and meteors struck, causing massive extinctions. Life has had a colorful and exciting last billion years, spawning diversity almost beyond our comprehension.
And yet, the giant steps of evolution remain back in the Precambrian. Its catalog of evolu- tionary innovations is long and impressive:
Energized elements from stardust formed simple organic molecules.
Building blocks chained together to form catalysts and self-replicating macromolecules.
Biochemical pathways evolved.
Protective yet permeable membranes enclosed the catalysts, replicators and their metabolic retinue.
Early prokaryotic cells “learned” to make ATP by splitting glucose.
www.ck12.org 518
Others began to harvest sunlight energy through photosynthesis.
Photosynthetic cyanobacteria produced vast amounts of “waste” oxygen, dramatically altering the Earth’s atmosphere.
The oceans rusted (iron ore deposits).
An ozone layer formed, shielding life from UV radiation.
The “O2 catastrophe” killed many anaerobic prokaryotes.
Still other prokaryotes “learned” to use the new O2 to release the energy remaining in carbohydrates products of glycolysis.
Endosymbiosis created eukaryotes, firmly establishing the three major evolutionary lineages, which yet today comprise the living world.
The timing and exact nature of most of these innovations is speculative; indeed, the first few may have been extraterrestrial and even deeper in time. They comprise perhaps the most important landmarks in the evolution of life, but the fossil record is sketchy due to prokaryote size, rock layer metamorphosis, and burial by more recent rocks.
Overall, we know remarkably little about Precambrian life. The Cambrian Period docu- ments the greatest flowering of life of all time, and gives its name - in a rather negative sense
to the 4 billion years of Earth history that preceded it. Before we dive into the famous Cambrian “explosion,” we will look more carefully at the last Eon of the Precambrian, which set the stage for this most famous burst of evolution.
The geologic record of the Proterozoic, the most recent eon of the Precambrian, is much better than that of the Archean and Hadean Eons before it. Accordingly, we know that supercontinents formed by collision and broke apart by rifting. The atmosphere changed dramatically with the addition of oxygen and a protective ozone layer. Glaciations covered much of the Earth with ice so extensively that it is known as the “Snowball Earth” during that period (Figure 11.27). Eventually, enough CO2 escaped from volcanoes to begin a period of global warming; melting opened a great variety of new niches. The severe restriction and subsequent opening of opportunities may have driven the later Cambrian explosion.
Within this dramatic environmental panorama, the three major lineages of life – Bacteria, Archaea, and Eukaryotes continued to diversify. Plant, animal, and fungal ancestors diverged as solitary cells. Gradually, some of these cells began to live in colonies. Within the colonies, primitive specialization among cells made certain tasks more efficient. The modern green alga, Volvox illustrates a comparable level of organization (Figure 11.28). The line between colonies and multicellular organisms is difficult to draw, but most scientists agree that true plants had evolved by about 1 billion years ago, and animals evolved about 100 million years later.


Figure 11.28: The green alga Volvox shows the multicellularity and early cell specializa- tion which probably characterized early colonial eukaryotes. Specializations include anterior sensory cells, asexual and two types of sexual reproductive cells, and coordination among flagellate cells. (50)
www.ck12.org 520
The fossil record shows that some eukaryotes had begun to reproduce sexually by a little over a billion years ago (Figure 11.29). Sexual reproduction was a major evolutionary innovation, producing more variety among offspring and thus more rapid adaptation to changing environments.
Figure 11.29: The evolution of sexual reproduction around 1 billion years ago increased variety among offspring, and may have increased rates of evolution (see Cell Division and Reproduction chapter). (45)
Near the end of the Precambrian - not until just over 600 million years ago, a unique assemblage of multicellular organisms left a fossil record which gives us our first glimpse of multicellular diversity – the Ediacaran biota (the name is taken from the hills in Australia where the first such fossils were found) (Figure 11.30).
Members of this community include:
some familiar organisms such as sponges, red and green algae, and bacteria
very few ancestors of modern animals
many unique disk, bag, and quilt like animals which do not resemble any modern animals
The origin and relatively rapid extinction of this entire group remain somewhat of a mystery. The oxygen atmosphere and/or an ice age may explain their initial radiation. Their abrupt and nearly complete disappearance may have resulted from unbalanced predation, grazing, or competition, or yet another environmental crisis such as supercontinent breakup, changes in ocean chemistry, and/or rising sea levels. Whatever the causes, most species disappeared by the end of the Precambrian, about 542 million years ago. The Ediacarans appear to have been an early multicellular, dead-end branch on the bush of life. Their extinction, however,

Figure 11.30: Spriggina (top), an Ediacaran fossil, may be an ancestor of the trilobites. Charnia (bottom), the first accepted complex Precambrian organism, is more typical of the Ediacaran biota – it is difficult to show relationships to any modern species. (22)
appears to have paved the way for a spectacular evolution of much more familiar life, which marks the beginning of the modern Phanerozoic Eon: the Cambrian explosion.
The Paleozoic era of the current, Phanerozoic Eon is the first concrete chapter of life’s history (Figure 11.31). Abundant fossils, clearly related to modern animals, plants and fungi, illuminate the path of evolution beginning with its first Period, the Cambrian, 542 million years ago. However, the sudden appearance of such variety presents yet another puzzle in the story of life: how did roughly 50 major groups of organisms evolve so rapidly, without apparent ancestors? The abrupt emergence of so many phyla has given this period
www.ck12.org 522
in geologic time its nickname, the Cambrian explosion, but its causes remain hypothetical. As for the Ediacaran radiation, major environmental changes have been proposed but not convincingly documented. A major geologic event of the Paleozoic is the amalgamation of the supercontinent Gondwana, but it does not seem to explain the extent of the increase in Cambrian diversity. Perhaps life itself was responsible: a “critical mass” of development could have opened up new body pattern options, or more kinds of life opened more kinds of ecological niches. Whatever the cause, the evidence shows that nearly all modern animal phyla, including our own chordate phylum, are represented in this diversity of life. Among the most common and famous are reef-building sponges and arthropods, known as trilobites (Figure 11.32). Both were diverse and abundant during the Cambrian but later became extinct. However, the phyla they represent persist today.
A major extinction marks the boundary between the Cambrian and Ordovician Periods 488 million years ago (Figure 11.33). In warm, shallow continental seas, Ordovician life rebounded:
A great diversity of new invertebrates swam the seas.
Liverworts may have been the first green plants to appear on land (Figure 11.34).
The first fish, jawless and bony-plated ostracoderms, swam slowly along shallow sea bottoms.
About 444 million years ago, a sharp drop in atmospheric CO2 led to glaciation and ended the long stable period of warm seas. The Ice Age affected marine genera severely; up to 60% disappeared! This major extinction marks the end of the Ordovician and the beginning of the Silurian Period.


www.ck12.org 524
During the Silurian, the glaciers retreated. Melting icecaps raised sea level, yet a new supercontinent, Euramerica, formed near the equator. In a long, stable greenhouse phase, warm shallow seas covered extensive equatorial landmasses, opening tropical habitats on land and in water:
Reef-building corals and sea-scorpions evolved.
The first jawed fishes joined armored jawless fishes and many invertebrates.
Vascular plants solved the problem of carrying water into the air.
Arthropods such as millipedes followed the plants onto land.
The Silurian ended about 416 million years ago with a minor extinction, which may have been due to an asteroid impact or increasing glaciation.
During the Devonian Period, terrestrial life expanded to include forests of clubmosses, horsetails, ferns, and the earliest seed-bearing plants and trees (Figure 11.35).
Seeds allowed plants reproduce on dry land in the same way that shelled eggs would later help animals. Insects appeared, although they were wingless at first.
Squid-like animals and ammonite mollusks became abundant.
Lobe-like fins allowed some fish to lift their heads above water and breathe air in oxygen-poor waters.
About 360 million years ago, extinction struck over 20% of marine families and over 50% of all genera, ending the Devonian. One hypothesis suggests that the greening of the con- tinents absorbed CO2 from the atmosphere, reducing the greenhouse effect and lowering temperatures.
Extensive coal deposits, fuel for our Industrial Revolution, characterize rocks of the Car- boniferous Period which followed. Coal developed from new bark-bearing trees in widespread lowland swamps and forests. Fallen trees were buried without decaying – perhaps because animals and bacteria had not yet evolved digestive enzymes that could break down the new molecule, lignin, in the wood. Burial of carbon lead to a corresponding buildup of oxygen in the atmosphere; O2 at the time was an all-time high of 35% (compared to 21% today).
Abundant oxygen probably encouraged evolution, especially on land.
As illustrated in Figure 11.36:
Giant insects took to the air.
Vertebrates moved to land; amphibians were far larger and more abundant and diverse than today.
The shelled egg allowed early reptiles to reproduce on land without drying out the embryo.
www.ck12.org 526
Early gymnosperms, reproducing with pollen rather than sperm, colonized dry land.
Toward the end of the Carboniferous, the climate cooled. Glaciation and extinction mark the border between the Carboniferous and the last period of the Paleozoic Era, about 300 million years ago.
The Permian is best known for the dramatic event which ended not only the period but also the entire Paleozoic Era – an extinction of 95% of the then-living world. If we look more closely at the effects of continental geography on climate, perhaps we can begin to understand not only that massive extinction, but also the major events in evolution which preceded it. During the Permian, all the major landmasses of earth combined into a single supercontinent, known as Pangaea (Figure 11.37). As for today’s continents, much of the interior would have been dry with seasons of temperature change, because the oceans’ moderating effects were too distant. Pangaea’s size may have exaggerated this continental climate of seasons and drought. Three major groups of animals and plants evolved in response to Pangaea’s extensive arid niches.
Reptiles, with claws, scaly skin, and shelled eggs, diversified, foreshadowing Mesozoic dinosaurs.
Cycads and other gymnosperms, with cuticle-covered leaves to limit water loss and cones to bear seeds, dominated forests.
Insects evolved entire life cycles on dry land; beetles and flies navigated land and air.
At the end of the Permian, an estimated 99.5% of individual organisms perished. Several factors may have contributed, and one factor relates again to Pangaea. Marine biodiversity is greatest in shallow coastal areas. A single continent has a much smaller shoreline than mul- tiple continents of the same size. Perhaps this restriction of marine habitats contributed to the drastic loss of species, for up to 95% of marine species perished, compared to “only” 70% of land species. Another factor might have been massive basalt flow attributed to the time, which could have increased CO2 levels to precipitate global warming. Some scientists invoke extraterrestrial causes: a huge meteorite crater discovered in 2006 in Antarctica and dated to between 100 and 500 million years ago could represent an impact which darkened skies, decreased sunlight, and shut down photosynthesis. Although the cause remains unknown, fossils clearly document the fact of Earth’s most devastating extinction. The event closed the Paleozoic Era, and inevitably opened the door to a new burst of life in the Mesozoic.
Following the “great dying” at the end of the Permian, a resurgence of evolution in the Mesozoic established the basis of modern life (Figure 11.38). The continents, which began as one, broke apart and eventually shifted into their present configuration. Rifting encouraged speciation (Figure 11.39). Relatively stable warm temperatures contributed once again to great diversification among animals.
During the Triassic, early dinosaurs appeared on land as the archosaurs, in the ocean as ichthyosaurs, and in the air as pterosaurs (Figure 11.40). One line of reptiles gave rise to the first mammals and others to the earliest turtles and crocodiles. Seed ferns and conifers dominated the forests. Modern corals and fishes, and many modern insects, evolved. The Triassic gave way to the Jurassic with one of the most active periods of volcanism ever recorded. Pangaea began to break apart. The major extinction marking the border between these two Periods opened niches which made way for the Age of the Dinosaurs.
The Jurassic Period was the golden age of the large dinosaurs which lived amidst warm, fern-and cycad-filled forests of pines, cedars, and yews (Figure 11.41). Dinosaurs included widespread and huge herbivorous sauropods, smaller predatory theropods, stegosaurs, and
www.ck12.org 528
pterosaurs. Ichthyosaurs and plesiosaurs thrived in the oceans. Ammonites, sea urchins, and starfish were abundant invertebrates. The first birds and lizards appeared. One of the most famous transition fossils, Archaeopteryx, with characteristics of both reptiles and birds, dates from this Period (Figure 11.42). During the Jurassic, the supercontinent Pangaea broke apart into Laurasia and Gondwana.


Figure 11.42: One of the most famous of all transitional fossils is Archaeopteryx, “ancient wings.” The fossil dates back to the Jurassic. Both reptilian features (teeth and claws) and avian features (wings and feathers) are clear. (34)
Flowering plants first appeared in the Jurassic, but dominated the last, Cretaceous Period
of the Mesozoic.
www.ck12.org 530
New kinds of insects coevolved with the flowering plants, serving as their pollinators.

An early example of this coevolution is the magnolia, which developed flowers to attract – and withstand feeding damage from - beetle pollinators. Bees first appeared during the Cre- taceous, and figs evolved unusual flower-fruits in concert with tiny wasp pollinators (Figure 11.43).
Primitive birds arose from reptilian ancestors and soon out-competed many of the pterosaurs.
All three major groups of mammals – monotremes, marsupials, and placentals – became established, but remained small.
In part because a huge sea (the Tethys) formed an east-west connection between the oceans, Cretaceous climate was uniformly warm; even the poles lacked ice. In response, warm- adapted plants and dinosaurs expanded to within 15 degrees of the poles. Dinosaurs reached a peak of diversity and size (Figure 11.44 and Figure 11.45).
Titanosaurs, including possibly the largest of all the dinosaurs, the 100-ton Argenti- nosaurus, were the dominant herbivores. A single Argentinosaurus vertebra was 1.3 meters long, and its tibia would have been as tall as some humans. Fossilized eggs, con- taining embryos with skin, indicate that titanosaurs were colonial nesters. Fossilized


Figure 11.45: Moderate climate worldwide during the Cretaceous encouraged great size and diversification among dinosaurs. The herbivorous titanosaur, Argentinosaurus (above) may have been the largest of all the dinosaurs, weighing in at up to 100 tons. Gigantosaurus (below) probably preyed upon titanosaurs such as Argentinosaurus, but weighed “only” 5.2 tons, and despite a bath-tub-sized skull, operated on a brain the size of a banana. (27)
www.ck12.org 532
dung shows they ate cycads and conifers, but also palms and the ancestors of rice and bamboo; some scientists suggest that dinosaurs and grasses coevolved like insects and flowering plants.
One of the largest predatory dinosaurs,Giganotosaurus, weighed “only” 5.2 tons, but in length surpassed Tyrannosaurus rex by two meters (six feet). Giganotosaurus’ skull was the size of a bathtub, but its brain was the size and shape of a banana! What
were they thinking?
The dramatic extinction of all dinosaurs (except the lineage which led to birds) marked the end of the Cretaceous. Dinosaurs had begun to decline earlier, perhaps due to reduction in atmospheric oxygen and global cooling. A worldwide iridium-rich layer, dated at 65.5 million years ago, provides evidence for an additional, more dramatic cause for their ulti- mate extinction. Iridium is rare in the Earth’s crust, but common in comets and asteroids. Scientists correlate this layer with a huge crater in the Yucatan and Gulf of Mexico. A colli- sion/explosion between the Earth and a comet or asteroid could have spread debris which set off tsunamis, altered the climate (including acid rain), and reduced sunlight 10-20%. A con- sequent reduction in photosynthesis would have caused a drastic disruption in food chains. Some scientists believe that volcanism also contributed to the “K-T” (Cretaceous-Tertiary) extinction, but most agree that “an impact event” was at least a major cause (Figure 11.46). The massive extinction and sharp geologic line led geologists to define the end of the Mesozoic and the beginning of our modern Era, the Cenozoic, with this event.

Neogene and Quaternary (Q) Periods share part of the Pliocene Epoch (Pl). Pleistocene
and Holocene (H) Epochs complete the Quaternary Period. Divisions in this part of the

Time Scale are debated and may change.
The Cenozoic Era brings the history of life into the present, but not without drama, mystery, and the looming possibility of a “Sixth Extinction” (Figure 11.47) You probably know the basic story: mammals took over where dinosaurs left off, branched to form primates, moved to the grasslands, became human-like, survived the ice ages, and the rest is – literally
history. Let’s look at some of the major events, focusing not only on our immediate ancestors but also on the world in which they evolved. More detailed stories of the evolution of humans and other groups will be told in later chapters.
Seven Epochs comprise the Cenozoic Era, with the Holocene continuing up to today. “Ter- tiary” refers to the 64 million years and five epochs before the Quaternary Period, well known for its recent ice ages and recognizable humans. Tertiary and Quaternary periods could be called suberas, but current organization of the Cenozoic segment of the Geologic Time Scale is the subject of current debate; it may well change.
The Paleocene Epoch provided a worldwide warm, humid climate for the rapid evolution which followed the extinction of the dinosaurs (Figure 11.48). Many plants, herbivores, and carnivores had disappeared because they depended on photosynthesis, but omnivores, insec- tivores, and scavengers – which included many mammals and birds – survived because their food sources actually increased. Mammals radiated into the ecological niches opened up by the extinction of herbivores and carnivores, and larger species, up to bear- or hippopotamus- sized, began to appear In equatorial regions, the first recognizably modern rain forests ap- peared, and south of the equator, hot arid regions provided niches for new groups of plants, including cacti.
Volcanism or a massive release of methane gas trapped in the oceans may have triggered one of the most rapid global warming events ever measured at the beginning of the Eocene, 56 million years ago. CO2 from either volcanism or oxidation of methane would have caused the oceans to become more acidic, and Earth’s temperatures to rise. Warm temperatures allowed forests of dawn redwood, swamp cypress, and palms to extend toward both poles. In the interiors of the continents, seasonal temperature and moisture variations led to the evolution of grasses, expansive savannas and deciduous forests. Within these new ecosystems, modern mammals with specialized teeth evolved. Probably due to high temperatures, these mammals were smaller than those who preceded them – or those who followed:
www.ck12.org 534
Figure 11.48: During the Paleocene, mammals and birds invaded ecological niches formerly occupied by the dinosaurs. Mammals included monotremes (A), marsupials, and hoofed placentals (B). Modern sharks (C) patrolled the seas. Birds included the giant flightless Gastornis (D). (53)
Horses and tapirs evolved in North America, and rhinoceros evolved in Asia.
Primates, with their long arms and legs and grasping hands and feet, appeared.
Mammals returned to the sea; Basilosaurus was an ancestor of today’s whales.
At the beginning of the Eocene, Australia was still connected to Antarctica, but when they broke apart, ocean currents changed and cooling began in earnest, foreshadowing the ice ages to come. Tundra ecosystems developed near the poles. Falling sea levels, a land bridge immigration of mammals from Asia to North America, and perhaps several impact events led to an extinction which marks the end of this epoch.
As its name implies, the Oligocene Epoch produced a “few” new mammals, especially in grasslands and savannahs (Figure 11.49).
Pig-like entelodonts used massive skulls to crush bones of scavenged prey.
One of the largest land mammals of all time, the 18-foot, 15 ton Indricotherium, ate leaves from the tops of trees in the manner of a giraffe.
Horses, represented by Mesohippus remained small relative to today’s species.
Large terrestrial carnivores such as Hyaenodon, hunted mammals up to the size of sheep.
The rhinoceros-like Arsinoitherium wandered tropical rain forests and swamps.
Figure 11.49: The Oligocene produced fewer new mammals than the Eocene; most were adapted to grasslands. Mesohippus (A) showed small steps toward modern horses. Hyaen- odon (B) had the large, sharp teeth of a carnivore. Elotherium (C) was a piglike scavenger, and Arsinoitherium (D) was a large relative of elephants and hyraxes. Perhaps the largest land mammal of all time resembled an overweight giraffe; Indricotherium (E) weighed up to 15 tons and reached 18 feet in height. (31)
www.ck12.org 536
By the beginning of the Miocene Epoch 23 million years ago, the continents had almost assumed their current configuration, except that North and South America did not connect. Oceans continued to cool, ice caps expanded at the poles, and consequently the climate dried. Grasslands, needing less rain, replaced forests, and large herbivores coevolved with the grasses. Modern mammals, including wolves, beaver, deer, camels, seals, dolphins, and porpoises, evolved. Up to 100 species of apes lived throughout Africa, Europe, and Asia. Almost all modern bird groups were represented.
The Earth’s climate continued to cool into the Pliocene, the epoch in which hominids first appeared. Seasons became more pronounced; deciduous forests and grasslands replaced trop- ical forests, and coniferous forests and tundra expanded. Large mammals, such as browsing mastodons and grazing mammoths, roamed the grasslands and tundra. Into this setting walked Australopithecines, such as Lucy who share common ancestry with humans. Fossil footprints dated as 3.7 million years old establish Australopithecenes as bipedal – perhaps the first apes to walk upright (Figure 11.50). Later Pliocene hominids included two mem- bers of our own genus, Homo rudolfensis and Homo habilis. During this epoch, falling sea levels exposed two land bridges which allowed important migrations.
Figure 11.50: Lucy (D) is one of the most complete fossils of Australopithecus afarensis, a human relative also known for fossil footprints which establish an upright posture. Note that the brown bones are Lucy’s; others have been added to restore her skeleton. Australo- pithecines coexisted (but not necessarily on the same continent!) with browsing mastodons (A), grazing mammoths (B), and giant herbivorous sloths (C). (14)
One allowed horses, mammoths, mastodons and more to migrate between Asia and North America.
A second allowed North American placental mammals, such as giant sloths, armadillos, and sabertooth cats, to migrate to South America. Placentals eventually out-competed all of South America’s marsupials except the opossum.
Repeated glaciations define the Pleistocene Epoch. Glaciation tied up huge volumes of water in ice packs; rainfall was less, because evaporation was less. Deserts were relatively dry. During interglacial periods, huge inland lakes and rivers held or carried the melt waters, and coastal flooding reduced land area. During the four major glaciations, these severe climate changes stressed animals and plants, encouraged the evolution of large animals (the Pleistocene megafauna), and forced life toward the equator.
Figure 11.51: That Homo erectus (A), and later (or in other parts of the world) Homo habilis, hunted mammoth (B) is shown by fossil evidence 1.8 million years old. Woolly mammoths (C), specially adapted to cold climate, were probably hunted, as well, as humans spread throughout the world. Nearly 40 woolly mammoth remains have been found preserved in permafrost, complete with soft tissue and DNA. To date, mitochondrial DNA has been sequenced. The calf (D) measures 2.3 meters (8 feet) long. A predatory competitor to humans was the saber-tooth tiger, (E). (52)
Some adapted to the cold: the Woolly Mammoth grew thick, shaggy hair oiled by abundant sebaceous glands, a layer of fat beneath the skin, smaller ears, and even a convenient flap to cover the anus, keeping out the cold. Mammoth teeth ground
www.ck12.org 538
tough tundra grasses, and their long, curved tusks may have helped to clear snow. Permafrosts have preserved nearly 40 mammoth remains, including soft tissues, and scientists actually hope to be able to recreate its genome; mitochondrial DNA for one species has already been sequenced! Using this sequence as a molecular clock, scientists calculate that mammoths diverged from African elephants about 6 million years ago, roughly the same time that humans diverged from chimpanzees.
Saber-tooth cats used dagger-like teeth to cut their prey’s windpipe and jugular veins, causing death by bleeding. Many saber-tooths have been found in the LaBrea Tar Pits in southern California, where they had tried to feed on mammoths trapped before them in the sticky tar/asphalt.
Homo erectus, the dominant hominid during the Pleistocene, migrated throughout
Africa, Europe, and Asia, giving rise to a number of variations of hominids. Although Homo erectus was probably the first hominid to leave Africa, the species may not have been a direct ancestor of humans. Pleistocene hominids were hunter-gatherers; evidence dated at 1.8 million years ago supports their consumption of mammoth.
A major extinction of Pleistocene megafauna continued into the Holocene. Some attribute the extinction to changing climate or disease, but others have connected the migrations of humans to each continent’s time of extinction The “overkill” theory suggests that humans hunted large animals with too much success. Agreement is not yet universal, but most scientists admit the evidence is strong.
The current Holocene Epoch began 11,550 years ago (about 9600 B.C.) with the retreat of the Pleistocene glaciers. During the Holocene, melting ice has raised sea level over 180 meters (600 feet). Geologists believe that we are currently experiencing an interglacial warming, and that glaciers will return – unless continued human burning of fossil fuels raises CO2 levels to bring about global warming. All of human civilization has occurred within the Holocene; Homo sapiens have passed through Mesolithic, Neolithic, and Bronze Age civi- lizations. Human evolution will be discussed in more detail in a future chapter, but here we will examine the possibility that humans are currently causing a mass extinction which some compare to the Permian. Many would include the Pleistocene megafauna in this “Sixth Extinction,” citing the “overkill theory” data in Figure 11.52. Some even call the period of time from that loss to the present the “Anthropocene epoch” to describe the major impact humans have had on the planet and its life. Human population has surpassed 6.6 billion, and over-fishing, climate change, industrialization, intensive agriculture, and clearance of grasslands and rainforests contribute to a startlingly high loss of life.
Paleontologists estimate that background extinction rates throughout most of life’s history averaged between 1 and 10 species per year (Figure 11.53) . The present rate of extinction is thought to be 100 to 1000 times ”background” rates, suggesting that the number of species which currently disappear each year could exceed 1,000! Biologist E.O. Wilson has predicted that current rates will result in the loss of over half of life’s biodiversity within the next one hundred years. In contrast, Earth’s shortest previous extinctions spanned several hundred thousand to several million years, and evidence for cause is entirely geological in nature. No
www.ck12.org 540
other species has influenced the Earth and its life as powerfully as Homo sapiens.
Others say there is ample evidence – evidence discussed in this lesson – to show that extinc- tion is a natural phenomenon which has occurred repeatedly throughout the history of life on Earth. They point to the recoveries – indeed, radiations – which filled vacated ecological niches after each event.
However, those who are concerned about the current extinction wonder whether or not humans will be one of the species to become extinct. Because we are the only surviving members of our family, recovery or radiation would not be an option.
What do you think?
After 3 billion years, life was unicellular but included all 3 major lineages: Bacteria, Archaea and Eukaryotes; all of multicellular evolution occurred within the last billion years.
Plant, animal, and fungal ancestors diverged as solitary cells.
Colonies of eukaryotic cells and specialized cells evolved; Volvox illustrates this level of evolution.
Sexual reproduction appeared a little over 1 billion years ago, providing more variation for natural selection.
By about 1 billion years ago, true (multicellular) plants emerged, and 100 million years later, true animals.
600 million year old Ediacaran fossils illustrate diverse early animal life, but few are related to modern animals.
The Cambrian explosion was the sudden appearance of great diversity in animals, plants, and fungi clearly related to modern species, due to lower O2, global warming, plate tectonics, and a critical mass of biotic change.
During the Ordovician, liverworts became the first land plants, and jawless, bony- plated fish joined a variety of invertebrates in warm seas.
Ferns and the first seed plants forested the land in the Devonian; in shallow seas, jawed fish evolved lobed fins.
Extensive coal deposits and an all-time high level of atmospheric O2 characterized the Carboniferous Period. Giant insects, early gymnosperms with pollen, and vertebrates with shelled eggs colonized dry land.
During the Permian, all the major landmasses of earth combined into a single super- continent, Pangaea. A continental climate of seasons and drought favored reptiles, gymnosperms, and insects such as beetles. The Permian ended with the most massive extinction of all time; 99.5% of all species disappeared, opening the door for a new radiation of species in the Mesozoic.
The Permian extinction, stable temperatures and continental breakup created niches www.ck12.org 542
for a great radiation of life in the Triassic Period. Reptiles diversified – on land as the archosaurs, in the air as pterosaurs, and in the seas as ichthyosaurs. Other reptilian lines gave rise to early turtles, crocodiles, and finally, mammals and birds.
During the Cretaceous, primitive birds began to radiate and out-compete the pterosaurs; dinosaurs reached their largest size and greatest diversity.
Record-high temperatures during the Eocene made way for hoofed animals and pri- mates within grasslands, savannas, and deciduous and coniferous forests.
Australopithecus, perhaps the earliest, upright hominid, appeared during the Pliocene.
Global cooling and glaciation led to a drop in sea level, exposing two land bridges which allowed important animal migrations.
Cycles of glaciation stressed Pleistocene animals and plants; some, like the woolly mammoth, adapted to cold and others, like Homo erectus, the dominant hominid, migrated throughout Africa, Europe, and Asia.
As the human population climbs above 6.6 billion, we may be causing a Sixth Extinc- tion of life on Earth.
What major evolutionary steps followed the evolution of the first eukaryotic cell during the late Precambrian to set the stage for the “Cambrian explosion?”
List the global environmental factors which influenced the evolution of multicellular life.
Discuss and give examples of the relationships among the environmental factors you listed above. Include their major effects on the history of life.
Describe the conditions under which the dinosaurs emerged to dominate life on Earth, and identify the diversity of habitats and niches occupied by the dinosaurs during their “golden age.”
What famous example of coevolution began in earnest during the Cretaceous? Give two early examples.
Cite and evaluate the evidence for an “impact event” as the primary cause of the K-T extinction.
Analyze the emergence of mammals and birds as dominant land animals during the early Cenozoic.
How does the Cenozoic climate explain the emergence of grassland and tundra and their megafauna?
Give two examples of how land bridge formation can affect evolution.
Discuss the factors which are contributing to the current major extinction, and analyze your own response to E.O. Wilson’s description of the “Sixth Extinction.”
Mark Pagel, ed. The Oxford Encyclopedia of Evolution. New York: Oxford University Press, 2002.
Stephen Jay Gould, ed. The Book of Life: An Illustrated History of the Evolution of Life on Earth. New York: W.W. Norton, 1993.
Colleen Whitney, Kate Barton, David Smith, “The Paleontology Portal.” University of California Museum of Paleontology, Paleontological Society, Society of Vertebrate Paleontology, and US Geological Survey, 2003. Available on the Web at:
“Continental Drift Animation.” EduMedia-sciences, 2002-2007.Available on the Web at:
http://www.edumedia-sciences.com/a95_l2-continental-drift.html
Dave Smith, “Life Has a History – Level 2.” University of California Museum of Pale- ontology, 7/18/06. Available on the Web at:
http://www.ucmp.berkeley.edu/education/explorations/tours/intro/Intro5to12/ tour1nav.php
David Ulansey, “The Current Mass Extinction.” David Ulansey, last updated 3 July
2007. Available on the Web at:
Lexi Krock, “The Missing Link: A Brief History of Life.” Nova Online, last updated February 2002. Available on the Web at:
Richard Cowen, “History of Life, 4th edition Updates, References and weblinks.” UC Davis Geology Department, 3 March 2006. Available on the Web at:
Roger Perkins, “The Virtual Fossil Museum: Fossils across Geologic Time and Evolu- tion.” Available on the Web at:
Roy Caldwell and David Lindberg, “Understanding Evolution.” University of California Museum of Paleontology, 2007. Available on the Web at:
http://evolution.berkeley.edu/
Archaeopteryx One of the most famous transition fossils; has characteristics of both reptiles and birds.
Cambrian explosion The abrupt emergence of many new species during the Cambrian Period.
liverworts Among the first true plants; colonized land during the Ordovician. www.ck12.org 544
Jurassic Period The golden age of the large dinosaurs which lived amidst warm, fern-and cycad-filled forests of pines, cedars, and yews.
Lucy One of the most complete fossils of Australopithecus afarensis, a human relative also known for fossil footprints which establish an upright posture.
marsupials One of the three major groups of mammals.
monotremes One of the three major groups of mammals.
Pangaea A single supercontinent formed from all the major land masses of Earth; formed during the Permian.
placentals One of the three major groups of mammals.
trilobites Common arthropods which were diverse and abundant during the Cambrian Period.
The study of the history of life attempts to answer the age-old question: where did we humans come from? What are some of the answers our current knowledge gives us? What points are still missing?
To what extent has life itself influenced the history of life on Earth? Consider some specific effects certain kinds of life have had on climate, the atmosphere, and certain species.
At least some mammoth DNA has been preserved in permafrost. What do you think about the idea of re-creating animals such as the mammoth from the past – as fiction- alized in Jurassic Park?
Do you think extinction plays an essential role in evolution? Is it a negative or positive role?
Do you judge the Sixth Extinction to be an important problem? Do you think it is significantly different from earlier extinctions?
http://en.wikipedia.org/wiki/Image:Evolution_pl.png. GNU-FDL.
http://commons.wikimedia.org/wiki/File:Golden_Jackal_sa02.jpg. Public Domain,Public Domain.
Elin Whitney Smith. http://en.wikipedia.org/wiki/Image: Extinctions_Africa_Austrailia_NAmerica_Madagascar.gif. Public Domain.
Petit J.R. et al. http://commons.wikimedia.org/wiki/Image:Carbon_Dioxide_400kyr.png, http://commons.wikimedia.org/wiki/Image: Instrumental_Temperature_Record.png. (a)GNU-FDL, (b)GNU-FDL 1.2, (c)GNU-FDL 1.2.
http://commons.wikimedia.org/wiki/Image: Homo_erectus_tautavelensis.jpg. GNU-FDL,GNU-FDL.
http://en.wikipedia.org/wiki/Image:Phanerozoic_Biodiversity.png. GNU-FDL.
http://en.wikipedia.org/wiki/Image:Tortoise-Hatchling.jpg http://commons.wikimedia.org/wiki/Image:Tree_bark.jpg http://commons.wikimedia.org/wiki/Image:Lignin_structure.svg http://commons.wikimedia.org/wiki/Image:Dragonfly_ran-384.jpg. (a)GNU-FDL (b)GNU-FDL (c)GNU-FDL (d)GNU-FDL (e)GNU-FDL.
http://commons.wikimedia.org/wiki/Image:Endosymbiosis.PNG. GNU-FDL.
http://commons.wikimedia.org/wiki/Image: Louse_diagram%2C_Micrographia%2C_Robert_Hooke%2C_1667.jpg. Public Domain,Public Domain.
http://commons.wikimedia.org/wiki/Image:Asaphiscuswheelerii.jpg. GNU-FDL,GNU-FDL.
USGS. http://commons.wikimedia.org/wiki/Image:Laurasia-Gondwana.png. Public Domain.
http://commons.wikimedia.org/wiki/Image: Exponential_Decay_of_Nuclei_with_Halflife-de.svg. GNU-FDL.
(a)Jovan Zujovic, (b)Rama, (c)Postdlf, (d)Ethiopia. http://commons.wikimedia.org/wiki/Image:Mammoth_mg_2791.jpg, http://en.wikipedia.org/wiki/Image:Ground_Sloth.jpg, http://commons.wikimedia.org/wiki/Image:Lucyreconstructionlarge.jpg. (a)Public Domain, (b)CC-BY-SA 2.0 France, (c)GNU-FDL, (d)GNU-PBL.
www.ck12.org 546
http://commons.wikimedia.org/wiki/Image:Fishapods.jpg http://commons.wikimedia.org/wiki/Image:Devonianscene.jpg. CC-BY-SA 2.5,GNU-FDL,Public Domain.
Giovanni Dall’Orto. http://commons.wikimedia.org/wiki/Image:
9121_-_Milano%2C_Museo_storia_naturale_-_Scipionyx_samniticus_-_Foto_ Giovanni_Dall%27Orto_22-Apr-2007a.jpg. The photographer of this file allows anyone to use it for any purpose, provided that the copyright holder is properly attributed. Redistribution, derivative work, commercial use, and all other use is permitted..
http://commons.wikimedia.org/wiki/Image:Edmontosaurusskin.jpg http://en.wikipedia.org/wiki/Image: ClaytonLakeStateParkDinosaurFootprint.jpg http://commons.wikimedia.org/wiki/File:Dinosauriereier_mongolei.JPG http://commons.wikimedia.org/wiki/Image:Tar-bigpit.jpg http://en.wikipedia.org/wiki/Image:Mammothzu2.jpg. Public Domain,Public Domain,GNU-FDL,CC-BY-SA 2.5, The photographer (Buchanan-Hermit) of this file allows anyone to use it for any purpose, provided that the copyright holder is properly attributed. Redistribution, derivative work, commercial use, and all other use is permitted,GFDL.
http://commons.wikimedia.org/wiki/Image:Evolution_con_dis.png. GNU-FDL.
Hannes Grobe. http://commons.wikimedia.org/wiki/Image:Earth_clock_hg.png. CC-BY-SA 2.5.
NASA. http://commons.wikimedia.org/wiki/Image:KT_Impact2.jpg. Public Domain.
Versimilis. http://en.wikipedia.org/wiki/Image:Charnia_Spun.jpg. GNU-FDL, GNU-FDL.
Stephen Hudson. http://commons.wikimedia.org/wiki/Image:AntarcticaDomeCSnow.jpg. GNU-FDL.
NOAA. http://commons.wikimedia.org/wiki/Image:Nur04506.jpg. Public Domain.
http://commons.wikimedia.org/wiki/Image:Biostratigraphie_usgs.png. Public Domain.
http://commons.wikimedia.org/wiki/File: Giganotosaurus_AustMus_email.jpg. GNU-FDL,Public Domain.
NASA. http://commons.wikimedia.org/wiki/Image:Ordovician_Sea.jpg. Public Domain.
http://commons.wikimedia.org/wiki/Image:Lipid_bilayer_and_micelle.png. GNU-FDL.
http://en.wikipedia.org/wiki/Image:Darwins_first_tree.jpg. Public Domain. (31)
http://commons.wikimedia.org/wiki/File:Hyaenodon_Heinrich_Harder.jpeg, http://commons.wikimedia.org/wiki/Image:Elotherium.jpg, http://commons.wikimedia.org/wiki/Image:Arsinoitherium_hharder.png, http://en.wikipedia.org/wiki/Image:Baluchitherium.jpg. Public Domain, Public Domain, Public Domain, Public Domain, Public Domain,.
http://commons.wikimedia.org/wiki/Image:UreyMillerExperiment.jpg. CC-BY 2.5,Public Domain, NASA.
http://commons.wikimedia.org/wiki/Image:Eoraptor_lunensis.png http://commons.wikimedia.org/wiki/Image:
Human-eoraptor_size_comparison%28v2%29.png http:
//commons.wikimedia.org/wiki/Image:Ichthyosaur_mounted_skeleton.jpg. (a)GNU-FDL (b)GNU-FDL (c)CC-BY-SA 2.5 (d)GNU-FDL.
Ballista. http://commons.wikimedia.org/wiki/File: Archaeopteryx_lithographica_-_cast_of_Humboldt_Museum_specimen.JPG. GNU-FDL.
http://commons.wikimedia.org/wiki/Image:Fig.jpg http://commons.wikimedia.org/wiki/Image:Blastophaga_psenes.jpg http://commons.wikimedia.org/wiki/Image:Beetle_collection.jpg http://commons.wikimedia.org/wiki/File:Soldier_0875.JPG. Public Domain,GNU-FDL,Public Domain,GNU-FDL,GNU-FDL.
http://commons.wikimedia.org/wiki/Image:Stromatolites_in_Sharkbay.jpg. Public Domain,GNU-FDL.
Kieff. http://commons.wikimedia.org/wiki/Image:Pangaea_continents.png. GNU-FDL.
www.ck12.org 548
John Romanes. http://commons.wikimedia.org/wiki/Image:Homology.jpg. Public Domain.
NASA. http://commons.wikimedia.org/wiki/Image:Keplers_supernova.jpg. Public Domain, Public Domain.
Gerhard Boeggemann. http://commons.wikimedia.org/wiki/Image: Europasaurus_holgeri_Scene_2.jpg. CC-BY-SA 2.5.
http://commons.wikimedia.org/wiki/Image:Ammonite_Asteroceras.jpg http://commons.wikimedia.org/wiki/Image:Triceratops_Struct.jpg http://commons.wikimedia.org/wiki/Image:
Human-triceratops_size_comparison.png http://commons.wikimedia.org/wiki/Image:ROM-HadrosaurSkeleton.png. GNU-FDL,Public Domain,GNU-FDL,GNU-FDL,CC-BY-SA 2.5,GNU-FDL.
http://commons.wikimedia.org/wiki/Image:Phylogenetic_tree.svg. Public Domain.
Stannered. http://commons.wikimedia.org/wiki/Image:Sexual_cycle.svg. GNU-FDL.
http://commons.wikimedia.org/wiki/Image:Macroglossum_stellatarum.jpg. GNU-FDL.
http://flickr.com/photos/brechtdc/536200459/. CC-BY-SA.
http://commons.wikimedia.org/wiki/Image:Phylogenetic_tree.svg. Public Domain.
http://en.wikipedia.org/wiki/Image:Punctuatedequilibrium.png. Public Domain.
CK-12 Foundation, Ralf Wagner. http://commons.wikimedia.org/wiki/File:Volvox_aureus_3_Ansichten.jpg. GNU-FDL.
http://commons.wikimedia.org/wiki/Image: Green_River_UT_2005-10-14_2104.jpg. CC-BY-SA 2.5.
(a)Luna04, (b)Peter 80, (c)Eino Mustonen, (d)Woudloper, (e)Postdif. http://commons.wikimedia.org/wiki/Image:Hunting_for_mammoth.jpg, http://commons.wikimedia.org/wiki/File: Mammuthus_primigenius_St_Petersbu_2.JPG, http://commons.wikimedia.org/wiki/Image:Mammoth_calf.jpg,
http://commons.wikimedia.org/wiki/Image:Smilodon_californicus.jpg. (a)GNU-FDL, (b)CC-BY-SA-2.5, 2.0, 1.0, (c)GNU-FDL, (d)Public Domain, (e)GNU-FDL.
http://commons.wikimedia.org/wiki/Image:Mesonyx.jpg http://commons.wikimedia.org/wiki/File:C._amblyrynchus.JPG http://commons.wikimedia.org/wiki/Image:Gastornis.jpg http://commons.wikimedia.org/wiki/Image:Dixi-Diatryma.png. (a)GNU-FDL (b)Public Domain (c)Public Domain (d)GNU-FDL (e)Public Domain.
www.ck12.org 550
Identify important ideas Darwin developed during the voyage of the Beagle, and give examples of his observations that supported those ideas.
Recognize that scientific theories and discoveries are seldom the work of just one indi- vidual.
Describe prevailing beliefs before Darwin about the origin of species and the age of the earth.
Evaluate Lamarck’s hypothesis about how species changed.
Analyze the impact of Lyell’s Principles of Geology on Darwin’s work.
Evaluate the influence of Malthus’ ideas about human population on Darwin’s thinking.
Discuss the relationship between Alfred Russel Wallace and Charles Darwin.
Describe the general ideas of Darwin’s Theory of Evolution.
Use Darwin’s reasoning to explain natural selection as the mechanism of evolution.
Explain how natural selection results in adaptation to environment.
Recognize the importance of variation to species survival.
Relate the idea of differential survival to the concept of natural selection.
Interpret the expression “descent with modification.”
Discuss the concept of “common ancestry.”
Show how Darwin’s theory provides a scientific explanation for the fossil record.
Interpret Darwin’s theory as an example of the general principle that the present arises from the materials and forms of the past.
Charles Darwin’s Theory of Evolution represents a giant leap in human understanding. It explains and unifies all of biology – thousands of years of natural history from before Darwin’s time, as well as the 150 years of genetics, molecular biology, and even ecology since Darwin published the theory. It directs our responses to disease and our practice of agriculture. It enlightens conservation biology. It has the potential to guide our future decisions about biotechnology. Apart from science, the Theory of Evolution has dramatically changed how we think about ourselves and how we relate to the world. Because the theory has influenced so many aspects of human life, it is crucial that you understand it thoroughly.
The “Theory of Evolution” contains two major ideas:
The first is evolution itself.
1. Present life has arisen gradually from past life forms. The millions of species of plants, animals, and microorganisms that live on Earth today are related by descent from common ancestors.
The second describes how evolution happens.
1. Natural selection explains how the diversity of life has arisen through time.
The main goal of this lesson will be to clarify these ideas. The lesson will begin by exploring Darwin’s experiences. The ideas of others who influenced Darwin’s thinking will also be presented. Finally, the content and significance of the theory itself will be analyzed.
Captained by a 26-year-old Royal Navyman and carrying a 22-year-old “gentleman’s com- panion” who collected beetles competitively, His Majesty’s Ship Beagle set sail on one of the shortest days of the year 1831 to chart South American coastal waters. Alarmed by the suicides of his own uncle and the previous Beagle commander, Captain Robert FitzRoy had sought a social and educational equal to accompany him at dinner and in scientific endeav- ors throughout the anticipated two-year voyage. Charles Darwin, financed by his wealthy father, assumed the unpaid positions of the ship’s naturalist and captain’s friend.
Darwin resisted his family’s hopes that he become a doctor or clergyman. During the two years before he dropped out of medical studies, he was repulsed by the brutality of surgery but fascinated by natural history – field observations of plants, animals, rocks, and fossils. He observed marine mammals on the English coast, and learned taxidermy from a freed slave whose talk of rain forests ignited curiosity in Darwin. After his disappointed father switched him to a school of theology, Darwin again gravitated toward natural history,
www.ck12.org 552

Figure 12.1: The HMS Beagle carried 22-year-old Charles Darwin as an unpaid naturalist and “gentleman companion” for the ship’s captain. (17)
becoming a protégée of botanist John Steven Henslow in order to learn the popular pastime of competitive beetle collecting. He managed to pass his theology exams, but his interests continued to reflect his passion for natural history, including William Paley’s “argument for divine design in nature.” He had just postponed entry into the clergy in order to study geology – mapping rock layers in Wales – when he received the invitation to join FitzRoy on the Beagle.
Planned to last two years, the voyage shown in Figures 12.1 and 12.2, stretched to five years. Darwin spent over 3 years of this time on land, carefully observing rock formations and collecting animals, plants, and fossils (Figure 12.4). Throughout the journey, he used his observations to develop a series of ideas which later became the foundation for his the- ory of evolution by natural selection (Figure 12.5). A few of his ideas, observations, and experiences follow.
During the voyage of the Beagle, Darwin made a number of geological observations that helped form his theory. Rock and fossil formations that he observed suggested that continents and oceans had changed dramatically over time.
Darwin found rocks at a continental divide, 13,000 feet above sea level, which contained fossil seashells.
A river in Argentina rose gradually through a series of plateaus, which Darwin and FitzRoy interpreted as ancient beaches.
After experiencing a volcanic eruption and an earthquake in Chile, Darwin found a
Figure 12.2: The Beagles voyage continued for nearly five years, although original plans called for only two. Darwin spent over three years of that time on land, collecting plants, animals, and fossils, and developing his ideas about evolution and natural selection. (27)
bed of newly dead mussels, which the quake had lifted nine feet above the sea.
A petrified forest embedded in sandstone at 7,000 feet had been a sunken coastal woodland, buried in sand and then uplifted into mountains.
Near Lima, Darwin recognized coral atolls as the result of sinking volcanoes, with coral adding layer after layer to keep the living reef close to the sunlit surface, as shown in Figure 12.3.
During the voyage of the Beagle, Darwin made a number of observations of plants, animals, and fossils that helped him form his theory. Observations of tropical rain forests and many new plant, animal, and fossil species encouraged Darwin to reconsider the source of the vast diversity of life.
In Brazil, Darwin collected great numbers of insects – especially beetles!
Inland from Montevideo, Darwin dug up the hippopotamus-like skull of an extinct giant capybara.
www.ck12.org 554

After collecting his first marsupial in Australia, Darwin exclaimed that some people might think “’Surely two distinct Creators must have been [at] work.”

Figure 12.4: Marine Iguanas (left) and Blue-footed Boobies (right) were among the tremen- dous variety of new and very different plants and animals Darwin identified during the voyage of the Beagle. He developed his ideas about evolution and natural selection to explain the remarkable similarities and differences he had observed. (6)
During the voyage of the Beagle, observations of native cultures led Darwin to question the relationship between humans and animals and the development of civilizations.
Disgusted by the enslavement of blacks in Brazil, Darwin argued with FitzRoy so fiercely that the captain temporarily banished him from dining.
At the tip of South America, Darwin wrote “I could not have believed how wide was the difference between savage and civilized man: it is greater than between a wild and domesticated animal.”
Darwin described New Zealand Maoris as savage, in contrast to missionary-influenced Tahitians.
Jemmy Buttons, a South American native who had “been civilized” in England, chose to stay in South America rather than continue with the Beagle - to the great dismay of the Englishmen convinced of their civilization’s superiority.

Figure 12.5: Darwin’s encounters with native cultures influenced his thinking as much as his discoveries of fossils and new species. This painting was taken from original pictorial records of the Beagle voyage. (23)
www.ck12.org 556
Darwin also made a number of observations that implied gradual changes in both the Earth and in living organisms, as opposed to catastrophic changes, including:
Many inland sediments had clearly been deposited by quiet tides rather than catas- trophic floods.
Gauchos, cowboys of Argentina, helped Darwin find and excavate fossils of gigantic extinct mammals, including armadillos and one of the largest mammals of all time, the ground sloth Megatherium (Figure 12.6). Darwin recorded that these sediments bore no trace of a Biblical flood.

Figure 12.6: Darwin found two separate fossils of one of the largest mammals of all time, a giant ground sloth, Megatherium. He noted that they were found in sediments which had been deposited slowly over long periods of time, rather than suddenly as by a catastrophic flood. (31)
The distribution of life on island chains challenged the dogma of the unchangability of species. The Galapagos Islands are arguably where Darwin made his most influential observations. The Galapagos Islands are a group of 16 volcanic islands near the equator about 600 miles from the west coast of South America. Darwin was able to spend months on foot exploring the islands.
Darwin noted that locals could distinguish each island’s variation of Galapagos tortoise, shown in Figure 12.7. Surprisingly, he did not collect their shells, despite dining on the giant reptiles during the voyage.
A series of birds now known as the Galapagos (or Darwin’s) finches were also specific to certain islands. Darwin failed to label the locations in which he had collected these rather drab-looking birds, but fortunately, FitzRoy and the ship’s surgeon were more careful with their collections.
Darwin interpreted the different Galapagos mockingbirds as varieties, but wrote that if varieties were a step on the way to new species, “such facts (would) undermine the stability of Species.”

Figure 12.7: Like many seamen, Darwin and the crew of the Beagle dined on Galapagos tortoise, a convenient animal to carry live on long voyages. However, locals living on the islands claimed the tortoises varied according to the islands from which they came, and this idea later played an important role in Darwin’s thinking about the origins of species. (20)
Throughout the trip, Darwin shown in Figure 12.8, sent his mentor, Henslow, collections of plants, animals, insects, and fossils – many of which were previously unknown. While Darwin traveled, Henslow promoted his work by sharing his geological writings and fossils with renowned naturalists. By the time the Beagle returned to England in October of 1836, Darwin himself had been accepted as an established naturalist. His father set up investment accounts to fund his son’s career as a “gentleman scientist.” At that time, governments and universities did not fund scientific research, so only independently wealthy individuals could afford to practice pure science. This position gave Darwin the contacts, resources, and freedom he needed to develop his ideas into the theory of evolution by natural selection.
www.ck12.org 558

Figure 12.8: Darwin’s writings on geology and the collections of plants, animals, and fossils he sent back to England established his reputation as a naturalist even before he returned from his voyage. After his return, his father supported him as a “gentleman scientist,” allowing him to further develop the ideas inspired by his Beagle travels. (3)
Science, like evolution, builds on the past. Darwin’s theory was a product not only of his own intellect, but also of the times in which he lived and the ideas of earlier great thinkers. Some of these ideas colored Darwin’s perspective during his five years on the Beagle; many contributed to his thinking after the voyage. Not until 23 years after he returned to England did Darwin crystallize his thoughts and evidence sufficiently to publish his theory.
Before Darwin, most people believed that all species were created at the same time and remained unchanged throughout history. History, they thought, reached back just 6,000 years.
One of the first scientists to explore change in species was Jean Baptiste Lamarck. Lamarck believed that organisms improve traits through increased use, and then pass the improved feature on to their offspring. According to this idea of inheritance of acquired charac- teristics, giraffes have long necks because early giraffes stretched their necks to reach tall trees and then passed the longer necks on to their calves, as shown in Figure 12.9. This attempt to explain adaptation was popular during the 19th century, and undoubtedly in- fluenced Darwin’s thinking. Although Lamarck advanced the proposal that species change, evidence does not support inheritance of acquired characteristics. You can weight-train for years, but unless your children train as hard as you did, their muscles will never match yours! We will look later at Darwin’s explanation for giraffes’ necks.
Much as Lamarck questioned the dogma that species do not change, Charles Lyell challenged the belief that the earth was young. In Principles of Geology, he recorded detailed observa- tions of rocks and fossils, and used present patterns and processes as keys to past events. He concluded that many small changes over long periods of time built today’s landscapes, and that the earth must be far older than most people believed. Captain FitzRoy gave Darwin a copy of Principles of Geology just before the Beagle left England, and Darwin “saw through [Lyell’s] eyes” during the voyage. Darwin’s theory that present species developed gradually over long periods of time reflects Lyell’s influence.
The idea that natural laws, rather than miracles, govern life as well as geology grew during the early 19th century. Charles Babbage wrote that God had the power to make laws, which in time produce species. His close friend, John Herschel, called for a search for natural laws underlying the ”mystery of mysteries” of how species formed. Later, Darwin cited Herschel as “one of our greatest philosophers” and then said he intended ”to throw some light on the origin of species — that mystery of mysteries.”
Darwin’s idea that individuals in a population compete for resources came from reading Thomas Malthus. Malthus described a human “struggle for existence” due to exponential population growth and limited food. Darwin thought that animal and plant populations might have similarly limited resources. If so, offspring suited to their environment would be more likely to survive, while those less “fit” would perish.
Breeders of pigeons, dogs, and cattle inspired Darwin’s ideas about selection. By choosing www.ck12.org 560

which animals reproduced, breeders could achieve remarkable changes and diversity in a relatively short time. Variations in traits were clearly abundant and heritable. Darwin referred to selective breeding as artificial selection. His observations of how artificial selection worked helped him to develop his concept of natural selection (Figure 12.10).

Figure 12.10: The way in which animal breeding artificially selects desirable variations in- fluenced Darwin’s ideas of natural selection. The English Carrier Pigeon (left), the English Fantail (center), and the Fiary Swallow (right) have all “descended” from the common rock pigeon (Columbia livia), with the help of human breeders. (24)
One of the last individuals to influence Darwin’s theory was Alfred Russel Wallace, a nat- uralist whose work in Malaysia led him to conclusions similar to Darwin’s. In 1858 - over 20 years since the Beagle returned to England - Wallace sent Darwin a paper which de- scribed concepts nearly identical to Darwin’s ideas about evolution and natural selection. Lyell helped arrange a joint presentation to the Linnean society two weeks later. Darwin, shocked by the sudden competition, worked quickly to complete his book by the following year. Although both naturalists had independently come to the same conclusions, the ex- tensive evidence and careful logic Darwin presented in The Origin of the Species earned him the greater share of recognition for the theory of evolution by natural selection.
Standing on the shoulders of the giants who went before him, Darwin was able to see past the countless details of his beloved work in natural history to formulate a unifying theory to explain the diversity of life.
Darwin lived in an increasingly scientific society which had begun to accept the idea that universal ”laws” governed processes in nature - perhaps including life itself. Like Lamarck, Darwin understood that species change. With Lyell, he saw that the history of Earth and its life covered a vast amount of time. From his observations of animal breeding, he recognized that even within species, individuals showed variation in traits, and that the variations could be passed to offspring. Recalling Malthus, he knew that populations could produce far more offspring than the environment could support. He predicted that individuals with traits which suited the environment would survive and reproduce to pass their favorable traits to offspring, as shown in Figure 12.11. Those whose traits were less suited to the
www.ck12.org 562
environment would die. Just as humans select for breeding those cattle which produce more milk, he reasoned, nature (the limited environment) selects individuals which use resources most efficiently. Thus, he called his explanation of how species change natural selection.
Darwin defined natural selection as the ”principle by which each slight variation [of a trait], if useful, is preserved,” and he later regretted that he had not named it “natural preservation.” Today it is often defined as the process by which a certain trait becomes more common within a population. Let’s look once more at the parts of this process, and then we will consider its consequences.
Darwin did not know that genes made of DNA determine traits. Much later, scientists learned that mutations in DNA can change genes and produce variations in traits. However, his observations of animal breeding and his detailed studies of barnacles and orchids con- vinced him that small, heritable variations in traits were common among individuals within a species. Darwin probably recognized that sexual reproduction increased variety in off- spring. He expressed considerable concern that his own health problems might be heritable,
especially when his beloved daughter Annie grew ill and died. He believed that his marriage to his cousin may have contributed to his children’s weaknesses.
Malthus argued that human populations grow exponentially if unchecked, but that disease, starvation, or war will limit population growth eventually. High birth rates and high death rates were characteristic of human history. Darwin himself had ten children; three died before maturity. Darwin reasoned that all species had the capacity to grow. However, his observations showed that most populations remained stable due to environmental limits. He concluded that many offspring must die. The phrases overproduction of offspring and struggle for existence summarize this idea.
Although heritable variations appeared to be random, death, Darwin reasoned, was not. Offspring which, by chance, had variations which “fit” or adapted them to their environment would have a greater chance to survive to maturity and a greater chance to reproduce. Offspring without such adaptations were more likely to die. Thus, well-adapted individuals produce more offspring. Differential survival and reproduction is a cornerstone of natural selection.
Can an individual organism evolve? No. Though an individual organism can be better adapted to its environment, it still must mate with others of its species, so by definition, it is not a new species. It is just an individual with a better chance of survival in its environment. It is the accumulation of many adaptations that, over many generations, results in a new species.
Through chance variation, overproduction of offspring, and differential survival and repro- duction, the proportion of individuals with a favorable trait (or favorable phenotype) will increase. The result is a population of individuals adapted to their environment. It is the variation within a species that increases the likelihood that at least some members of a species will be adapted to their environment and survive under changed conditions.
It is important to note that natural selection is not directed or intentional. It depends on chance variations - due to genetic variations - and can work only with the “raw material” of existing species. Occasionally, variations which have no particular adaptive logic may
www.ck12.org 564
survive. However, the limits set by resources and environment usually mean an increase in traits which help survival or reproduction, and the loss of traits which harm them. Gradually, species change. Eventually, changes accumulate and a new species is formed.
Let’s compare natural selection to inheritance of acquired characteristics (Lamarck’s idea mentioned above). How would Darwin’s mechanism explain the long necks of giraffes?
Heritable variation: In the past, some giraffes had short necks, and some had long necks.
Overproduction of offspring: Giraffes produced more young than the trees in their environment could support.
Differential survival and reproduction: Because the long-necked giraffes could feed from taller trees, they were more likely to survive and produce more offspring. Short-necked giraffes were more likely to starve before they could reproduce.
Species change: The long-necked giraffes passed their long necks on to their calves, so that each generation, the population contained more long-necked giraffes.
Recall that Lamarck believed that giraffes could stretch their necks to reach tall trees, and pass their stretched necks on to offspring. If this were true, evolution would reward effort toward a goal. Darwin showed that evolution is not goal-directed. Instead, the environment reinforces variations which occur by chance.
Lyell studied the geology which surrounded him and saw that the environment had changed many times over a vast amount of time. Darwin studied the life across continents and saw, in addition to tremendous variation, that species had changed – in response to the changes in their environment – over that vast amount of time. Both proved, with careful observations and well-reasoned inferences, that the present arises from the past. Limited to our brief lifespans, we see today’s species as fixed. Darwin taught us how to see the relationships between them; to see that they developed from earlier, distinctly different species; to see that all of them - all of us - share common ancestors (Figure 12.12). The cartoons which showed Darwin as an ape (an example is shown in the next lesson) did a great disservice to his theory of evolution. Far too many people limit their understanding of evolution to the simple phrase that “we came from apes.” We humans share common ancestors not only with the great apes, but with ALL of life – blue whales, gazelles, redwood trees, saguaros, fireflies, mosquitoes, puffballs, amebas, and bacteria. As Darwin said in closing the Origin, “There is grandeur in this view of life.”
Darwin delighted in the great diversity of life, but also saw unity within that diversity. He saw striking patterns in the similarities and differences. Seeking an explanation for those patterns, he developed the concept of natural selection. Natural selection explains how today’s organisms could be related – through “descent with modification” from common ancestors. Natural selection explains the story told by the fossil record – the long history of life on Earth. Natural selection is a scientific answer (if only partial) to the old questions: Who are we? How did we come to be?

Figure 12.12: A sketch from Darwin’s Origin of Species, this ”Tree of Life” depicts his ideas of how today’s species (top row, XIV) have descended with modification from common ancestors. The theory implies that all species living today have a universal common ancestor
that we humans are related to all of Earth’s plants, animals, and microorganisms. (34)
In the light of natural selection, it is easy to see that variation – differences among individuals within a population – increases the chance that at least some individuals will survive if the environment changes. Here is a strong argument against cloning humans: if we were all genetically identical – if variation (or genetic variation) did not exist – a virus which previously could kill just some of us would either kill all of us, or none of us. Throughout the long history of life, variation has provided insurance that inevitable changes in the environmental will not mean the extinction of a species. Similarly, the diversity of species ensures that environmental change will not mean the extinction of life. Life has evolved (or, the Earth’s changing environment has selected) variation and diversity because they ensure survival. Causes of mutation may have pre-existed, but in a sense, life has embraced them. And sexual reproduction has evolved to add further to variation and diversity (as discussed in the Cell Division and Reproduction chapter).
Adaptations are logical because the environment imposes limits on organisms, selecting against those who do not “fit.” Adaptations arise through gradual accumulation of chance variations, so they cannot be predicted, despite the fact that they appear to be goal-directed or intentional. Adaptations relate to every aspect of life: food, water, oxygen, nutrients, shelter, growth, response, reproduction, movement, behavior, ability to learn. Adaptations connect organisms to the resources in their environments. You are born with your adapta- tions; they are not changes you make to fit yourself into an environment. If the environment changes, the adaptive value of some of your inherited characteristics may also change. Our human appetites for salt and fat, for example, may remain from our past, when fat and salt
www.ck12.org 566
were rare in our environment; now that they are easily available, we consume more than is good for us. Biologist E.O. Wilson believes adaptations reach every aspect of human life - that social, political, and even religious behaviors are rooted in our genes. Of course, we can learn – and learning allows us to adapt within our lifetimes to environmental change. The ability to learn is itself an adaptation – perhaps our greatest gift. But more and more, we are discovering that much of our behavior – including learning - is genetically programmed
a gift from our ancestors similar to vision and hearing, or breathing and digestion.
Darwin’s theory can be summarized in two statements All living species share com- mon ancestors, and
Natural selection explains how species change.
In this lesson, we have explored Darwin’s reasoning. In the next lesson, we will consider the abundant evidence which supports his ideas.
The Theory of Evolution has changed how we see ourselves and how we relate to our world.
The theory has two basic ideas: the common ancestry of all life, and natural selection.
Darwin studied medicine and theology, but he first worked as ship’s naturalist on the HMS Beagle.
During the 5-year voyage, Darwin spent over 3 years on land exploring new rocks, fossils, and species.
From his observations, Darwin developed new ideas which later formed the foundation of his theory.
Rock and fossil formations suggested that continents and oceans had changed dramat- ically.
Tropical rain forests encouraged Darwin to reconsider the source of the vast diversity of life.
Native cultures raised questions about the relationship between humans and animals.
Sedimentary rocks implied gradual, as opposed to catastrophic, changes in the earth and in life.
The distribution of life on island chains challenged the dogma of the immutability of species.
After he returned, his reputation as a naturalist and his father’s financial support allowed him to become a “gentleman scientist,” free to analyze his collections, formulate his theory, and write about both.
Like all scientific theories, Darwin’s was a product of both his own work and the work of other scientists.
Before Darwin, most people believed that all species were created and unchanging about 6000 years ago.
Jean-Baptiste Lamarck proposed that acquired characteristics could be inherited. Evi- dence did not support his mechanism for change, but Darwin shared his ideas of change in species.
Charles Lyell wrote that present rock formations have developed through gradual changes over long periods of time. Darwin applied his ideas to present life forms.
Observations of animal breeding helped Darwin appreciate the importance of heritable variations.
Malthus’ work showed that populations produce more offspring than the environment can support.
Charles Babbage and John Herschel believed that natural laws governed the origin of species.
Alfred Russel Wallace formulated a theory very similar to Darwin’s. Although they collaborated on a joint paper, Darwin’s clear and forceful Origin of Species earned him greater credit.
The two general ideas of Darwin’s Theory are evolution and natural selection.
The concept of natural selection includes these observations and conclusions:
By chance, heritable variations exist within a species.
Species produce more offspring than can survive.
Offspring with favorable variations are more likely to survive to reproduce.
Gradually, individuals with favorable variations make up more of the population.
Variation among individuals within species ensures that some will survive environmen- tal change.
Because some variations help survival in a specific habitat more than others, individuals having those variations are more likely to survive and reproduce.
This differential survival and reproduction results in a population which is adapted to its environment.
The result of natural selection is gradual change in species, and when enough changes have accumulated, new species form. This is “descent with modification.”
The idea that natural selection has led to the origin of all species, together with evidence from the fossil record, means that all existing species are related by “common ancestry.”
Evolution by natural selection explains the history of life as recorded in the fossil record.
Common ancestry explains the similarities, and natural selection in the face of envi- ronmental change explains the differences among present-day species.
Like Lyell’s Principles of Geology, Darwin’s Theory of Evolution supports the general principle that the present arises from the materials and forms of the past.
www.ck12.org 568
State 3 of the 5 ideas Darwin developed during the Voyage of the Beagle. For each idea, give and example of a specific observation he made which supports the idea.
Compare and contrast Darwin’s position as a “gentleman scientist” with today’s pro- fessional scientists.
What does the expression “standing on the shoulders of giants” say about Darwin and his Theory of Evolution? Support your interpretation with at least three specific examples.
Explain the importance of Lyell’s Principles of Geology to Darwin’s work.
Discuss the influence of animal breeding on Darwin’s thinking.
Clarify the relationship between Darwin and Alfred Russel Wallace.
Summarize in your own words the two basic ideas which make up Darwin’s Theory of Evolution.
Compare and contrast Lamarck’s and Darwin’s ideas using the evolution of the human brain as an example.
Why is it incorrect to say that evolution means organisms adapt to environmental change?
Why is it not correct to say that evolution means “we came from monkeys?”
http://www.literature.org/authors/darwin-charles/the-origin-of-species/
http://www.life.umd.edu/emeritus/reveal/pbio/darwin/darwindex.html
adaptation A characteristic which helps an organism survive in a specific habitat.
artificial selection Animal or plant breeding; artificially choosing which individuals will reproduce according to desirable traits.
inheritance of acquired characteristics The idea that organisms can increase the size or improve the function of a characteristic through use, and then pass the improved trait on to offspring.
law A statement which reliably describes a certain set of observations in nature; usually testable.
natural selection The process by which a certain trait becomes more common within a population, including heritable variation, overproduction of offspring, and differential survival and reproduction.
theory An explanation which ties together or unifies a large group of observations.
How might the Theory of Evolution help us to understand and fight disease?
What other aspects of medicine could benefit from an understanding of evolution?
How can evolution and natural selection improve conservation of species and their environments?
How would you put into words the ways in which evolution has changed the way we look at ourselves?
How do you think it has altered the way we relate to other species? To the Earth?
Consider the human brain. If Lamarck’s hypothesis about inheritance of acquired characteristics were true, how would your knowledge compare to your parents?
Clarify the significance of a scientific theory.
Recognize that Darwin supported his theory with a great deal of evidence, and that many kinds of evidence since his time have further strengthened the theory of evolution.
Describe how Darwin used the fossil record to support descent from common ancestors.
Compare and contrast homologous structures and analogous structures as evidence for evolution.
Give examples of evidence from embryology which supports common ancestry.
Explain how vestigial structures support evolution by natural selection.
Discuss the molecular similarities found in all species of organisms.
Describe how evolution explains the remarkable molecular similarities among diverse species.
Analyze the relationship between Darwin’s Theory of Evolution and more recent dis- coveries such as Mendel’s work in genetics and the molecular biology of DNA and protein.
Relate the distribution of plants and animals to changes in geography and climate.
Explain how biogeography supports the theory of evolution by natural selection.
Summarize the explanation given by both Darwin and Wallace for the distribution of few, closely related species across island chains.
www.ck12.org 570
You are probably aware that the concept of evolution still generates controversy today, despite its wide acceptance. In The Origin of the Species, Darwin mentioned humans only once, predicting, ”Light will be thrown on the origin of man and his history.” Nevertheless, some people immediately distorted its far-reaching message about the unity of life into near- sighted shorthand: humans “came from” monkeys (Figure 12.13).

In the last lesson, you learned that evolution relates all of life – not just humans and monkeys. In this lesson, you will learn that biological evolution, like all scientific theories, is much more than just an opinion or hypothesis, it is based on evidence.
In science, a theory is an explanation which ties together or unifies a large group of ob- servations. Scientists accept theories if they have a great deal of supporting evidence. In The Origin of the Species, Darwin took the time to compile massive amounts of fossil and biological evidence to support his ideas of natural selection and descent from common an- cestors. He clearly and effectively compared animal breeding (artificial selection), which was familiar to most people, and natural selection. Because Darwin provided so much evidence and used careful logic, most scientists readily accepted natural selection as a mechanism for
change in species. Since Darwin’s time, additional fossil and biological data and new fields of biology such as genetics, molecular biology, and biogeography have dramatically confirmed evolution as a unifying theory – so much so that eminent biologist Theodosius Dobzhansky wrote that “Nothing in biology makes sense except in the light of evolution.”
In this lesson, you can explore and evaluate for yourself the many kinds of evidence which support the theory of evolution by natural selection. You will also have the opportunity to appreciate the power of evolution to explain observations in every branch of biology.
Few would argue that dinosaurs roamed Earth in the past, but no longer exist. The fossil record is a revealing window into species that lived long ago. Paleontologists have care- fully analyzed the preserved remains and traces of animals, plants, and even microorganisms to reconstruct the history of life on Earth (see the History of Life chapter for more detail). Relative (rock layer position) and absolute (radioisotope) dating techniques allow geol- ogists to sequence the fossils chronologically and provide a time scale. Geology also reveals the environmental conditions of past species.
For many reasons, the fossil record is not complete. Most organisms decomposed or were eaten by scavengers after death. Many species lacked hard parts, which are much more likely to fossilize. Some rocks and the fossils they contained have eroded and disappeared. Moreover, much of evolution happens in the small populations that survive changes in en- vironmental conditions, so the chance that intermediates will fossilize is low. Nevertheless, the current record includes billions of fossils – over 300 million from Los Angeles’ LaBrea Tar Pits alone, and an estimated 800 billion in South Africa’s Beaufort Formation. Analysts have identified 250,000 species among these remains.
Although the fossil record is far more detailed today than in Darwin’s time, Darwin was able to use it as powerful evidence for natural selection and common descent. Throughout geo- logical history, species that appear in an early rock layer disappear in a more recent layer. Darwin argued that a species’ appearance recorded its origin, and that its disappearance showed extinction. Moreover, he noted remarkable similarities among structures in differing species, supporting common ancestry. Finally, he could often correlate environmental con- ditions with structures, supporting his idea that natural selection led to adaptations which improved survival within certain habitats.
As an example, let’s analyze a relatively complete set of fossils which record the evolution of the modern horse. Figure 12.14 sequences five species which show major evolutionary changes. The oldest fossil shows a fox-sized animal with slender legs and nearly vertical digits: Hyracotherium bit and chewed soft leaves in wooded marshlands. Geology and paleontology suggest that the climate gradually dried, and grasslands slowly replaced the marshes. Meso- hippus was taller, with fewer, stronger digits – better able to spot and run from predators, and thus more likely to survive and reproduce in the new grasslands. Merychippus was taller
www.ck12.org 572
still, and kept only one, enlarged digit – a hoof to run fast on the hard ground. By Pliohippus time, molar teeth had widened and elongated to grind the tough grasses. These fossils show gradual structural changes which correspond to changes in the environment. They appear to show a smooth, linear path directed toward the “goal” of the modern horse, but this is deceiving. These five fossils are merely “snapshots” of a bushy family tree containing as many as 12 genera and several hundred species. Some transitions are smooth progressions; others are abrupt. Together, they support natural selection and descent with modification from common ancestors.
The evidence Darwin presented in The Origin of Species included not only fossils but also detailed comparisons of living species at all life stages. Naturalists in Darwin’s time were ex- perts in comparative anatomy – the study of the similarities and differences in organisms’ structures (body parts). At different times during his life, Darwin studied the comparative anatomy of closely related species of marine mammals, barnacles, orchids, insectivorous plants, and earthworms.
Species which share many similarities are closely related by a relatively recent common ancestor. For example, all orchids share parallel-veined leaves, two-sided flowers with a “lip,” and small seeds (Figures A and B 12.15). Species which share fewer similarities, sharing only basic features, are related by relatively distant ancestor. The sundew, one of the insectivorous plants Darwin studied, shares leaves and petals with orchids, but the leaves are wide with branching veins and the flowers are radially symmetrical rather than two-sided (Figure C 12.15). The many species of orchids, then, share a recent common ancestor, but they also share a more distant ancestor with the sundew.
Similarities can show two different kinds of relationships, both of which support evolution and natural selection.
Similarities shared by closely related species (species who share many characteristics) are homologous, because the species have descended from a common ancestor which had that trait. Homologous structures may or may not serve the same function. Figure 12.16 shows the forelimbs of mammals, considered homologous because all mammals show the same basic pattern: a single proximal bone joins a pair of more distal bones, which connect to bones of the wrist, “hand,” and digits. With this basic pattern, bats build wings for their lives in the air, whales form fins for their lives in the sea, and horses, as we have seen, construct long, hoofed legs for speed on land. Therefore, homologous structures support common ancestry.
Similarities shared by distantly related species may have evolved separately because they live in similar habitats. These structures are analogous because they serve similar functions,
www.ck12.org 574
but evolved independently. Figure 12.17 compares the wings of bats, bird, and pterosaurs. Bats evolved wings as mammals, pterosaurs as dinosaurs, and birds from a separate line of reptiles. Their wings are analogous structures, each of which evolved independently, but all of which suit a lifestyle in the air. Note that although the wings are analogous, their bones are homologous: all three share a common but more distant vertebrate ancestor, in which the basic forelimb pattern evolved. Because analogous structures are independent adaptations to a common environment, they support natural selection.
Embryology is a branch of comparative anatomy which studies the development of verte- brate animals before birth or hatching. Like adults, embryos show similarities which can support common ancestry. For example, all vertebrate embryos have gill slits and tails, shown in Figure 12.18. The “gill slits” are not gills, however. They connect the throat to the outside early in development, but in many species, later close; only in fish and larval amphibians do they contribute to the development of gills. In mammals, the tissue between the first gill slits forms part of the lower jaw and the bones of the inner ear. The embryonic tail does not develop into a tail in all species; in humans, it is reduced during development to the coccyx, or tailbone. Similar structures during development support common ancestry.
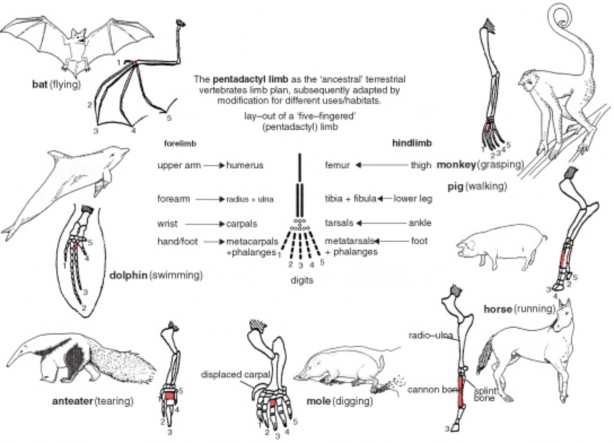
Figure 12.16: Homologous structures are similarities throughout a group of closely related species. The similar bone patterns in bat’s wings, dolphin’s flippers, and horse’s legs support their descent from a common mammalian ancestor. (13)
www.ck12.org 576
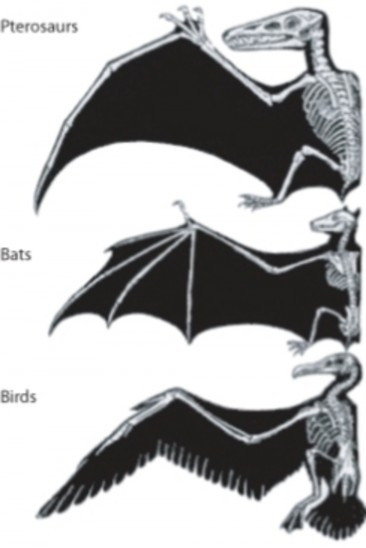
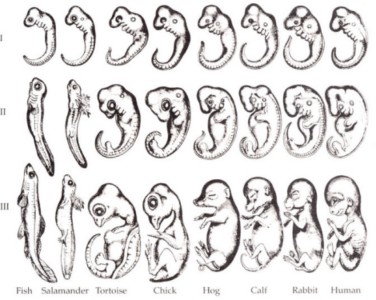
Figure 12.18: Comparative embryology reveals homologies which form during develop- ment but may later disappear. All vertebrate embryos develop tails, though adult humans retain only the coccyx. All vertebrate embryos show gill slits, though these develop into gill openings only in fish and larval amphibians. In humans, gills slits form the lower jaw and Eustachian tube. Many scientists consider developmental homologies evidence for an- cestry, although some embryologists believe that these particular drawings exaggerate the similarities. (8)
www.ck12.org 578
Structures which are reduced and perhaps even nonfunctional, such as the human tail and the human appendix, are considered vestigial structures. The tail, of course, functions for balance in many mammals, and the human appendix may have served digestive functions in herbivorous ancestors. Whales, which evolved from land mammals, do not have legs or hair as adults; both begin to develop in embryos, but then recede. Vestigial leg bones remain, buried deep in their bodies, shown in Figure A 12.19.
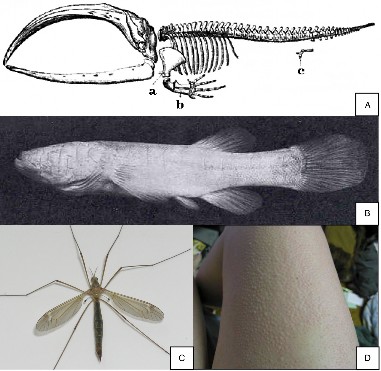
True flies have reduced the second pair of wings found in most insects to halteres for balance shown in Figure B 12.19. Cavefish lose both eyes and pigment, because both would require energy to build and are useless in the lightless habitat they have adopted shown in Figure C
12.19. You are probably very familiar with a fine example of a vestigial behavior: goosebumps raise the sparse hairs on your arms even though they are no longer sufficiently dense to insulate you from the cold by trapping warm air next to your skin; in most mammals, this reflex is still quite functional shown in Figure D 12.19. Most vestigial structures are
homologous to similar, functioning structures in closely related species, and as such, support both common ancestry and (incomplete!) natural selection.
Did you know that your genes may be 50% the same as those of a banana?
Unknown in Darwin’s time, the “comparative anatomy” of the molecules which make up life has added an even more convincing set of homologies to the evidence for evolution. All living organisms have genes made of DNA. The order of nucleotides – As, Ts, Cs, and Gs - in each gene codes for a protein, which does the work or builds the structures of life. Proteins govern the traits chosen (or not) in natural selection. For all organisms, a single Genetic Code translates the sequence of nucleotides in a gene into a corresponding chain of 20 amino acids. By itself, the universality of DNA genes and their code for proteins is strong evidence for common ancestry. Yet there is more.
If we compare the sequence of nucleotides in the DNA of one organism to the sequence in another, we see remarkable similarities. For example, human DNA sequences are 98-99% the same as those of chimpanzees, and 50% the same as a banana’s! These similarities reflect similar metabolism. All organisms have genes for DNA replication, protein synthesis, and processes such as cellular respiration. Although metabolic processes do not leave fossils, similar DNA sequences among existing organisms provide excellent evidence for common ancestry.
The differences in DNA sequences are even more intriguing. Many are single base sub- stitutions resulting from mutations accumulated through time. Assuming mutations occur randomly, the number of differences in bases between any two species measures the time elapsed since two organisms shared a common ancestor. This type of ”molecular clock” has confirmed traditional classification based on anatomy. Most scientists consider it sufficiently powerful to clarify or correct our understanding of evolutionary history. For example, human DNA differs 1.2% from chimpanzees, 1.6% from gorillas, and 6.6% from baboons; we can infer from this data that humans and chimpanzees share a relatively recent common ances- tor, and that the common ancestor we share with gorillas lived much longer ago. Figure
shows a cladogram depicting hypothetical evolutionary relationships constructed with this data. Similarities and differences in the sequences of amino acids in proteins support common ancestry in the same way, because they are determined by DNA.
Heritability and variation in traits are essential parts of Darwin’s theory of evolution by natural selection. Since he published The Origin of the Species, rediscovery of Mendel’s identification of genes and how they are inherited has confirmed Darwin’s ideas. Molecular biology has clarified the nature of genes and the sources of variation. Comparative analysis of DNA and proteins continues to give us an exquisitely detailed view of patterns of variation, common ancestry, and how evolution works.
www.ck12.org 580
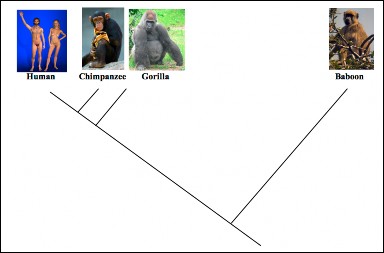
Australia, Africa, and South America occupy the same latitude, at least in part, and therefore have roughly the same climate. If plants and animals were distributed only according to their adaptations to habitat, we would expect the same species to occupy similar regions of these continents. However, the short-tailed monkeys, elephants, and lions in Africa differ significantly from the long-tailed monkeys, llamas, and jaguars of South America, and even more from the koalas, kangaroos, and Tasmanian devils of Australia. Biogeography studies the distribution of plants and animals and the processes that influence their distribution – including evolution and natural selection. Only geologic change and evolution can explain the distributions of many species, so biogeography is another kind of evidence for the theory of evolution.
Alfred Russel Wallace, who developed his own ideas of evolution and natural selection at the same time as Darwin, explained the distributions of many species in terms of changes in geography (such as formation of land bridges) and environment (for example, glaciations) and corresponding evolution of species. Figure 12.21 shows the six biogeographical regions he identified: Nearctic, Neotropical, Palaearctic, Ethiopian, Oriental, and Australian.
Let’s consider just the camel family as an example, shown in Figure 12.22 of how biogeog- raphy explains the distribution of species. Fossils suggest that camel ancestors originated in North America. Distant fossils show structural similarities which suggest that their de- scendants migrated across the Bering land bridge to Asia and across the Isthmus of Panama into South America. These two isolated populations evolved in different directions due to
differences in chance variations and habitat. Today’s descendants are llamas and guanacos in South America, and camels in Asia. Asian camels continued to migrate west into Africa, giving rise to two species – the dromedary in Africa, and the Bactrian in eastern Asia.
The distribution of some older fossils shows an opposite pattern; for example, fossils of a single species of fern, Glossopteris, have been found in South America, Africa, India, Antarctica, and Australia (Figure 12.23). Putting together many such distributions and a great deal of geologic data, Alfred Wegener showed that the continents were long ago united as Gondwanaland, and have since drifted apart. His theory of continental drift and its modern form, plate tectonics, help to further explain patterns of evolutionary descent in space and time.
Island biogeography studies archipelagos (oceanic island chains) as isolated sites for evo- lution. Both Darwin and Wallace used examples from isolated oceanic islands, such as the Galapagos and Hawaii, in their arguments for evolution and natural selection. Until hu- mans arrived, terrestrial mammals and amphibians were completely absent on these islands. Darwin and Wallace showed that the animals and plants which were present had blown or drifted from one of the continents, or had descended – with modifications which suited the new habitats – from one of the original colonists. Terrestrial mammals and amphibians, hav- ing no powers of dispersal across oceans (until humans came along), were understandably absent.
www.ck12.org 582
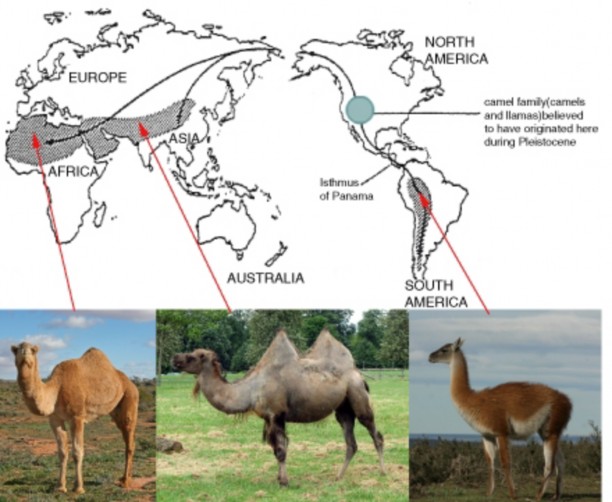
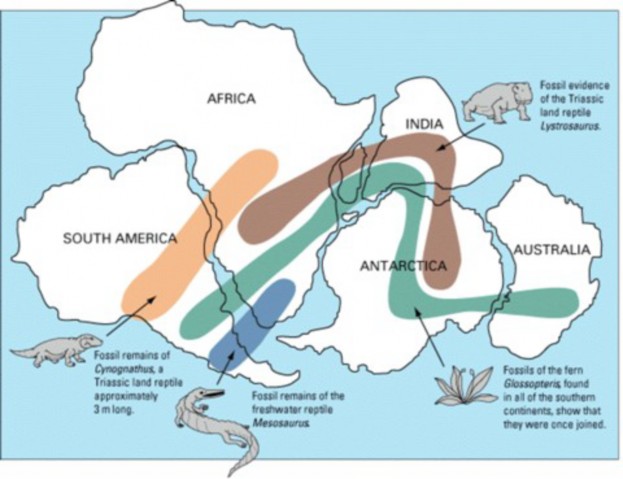
www.ck12.org 584
Only long after returning from his voyage did Darwin, with help from ornithologist John Gould, realize that the Galapagos birds he had collected but dismissed as uninteresting blackbirds, grosbeaks, finches, and a wren, were actually all closely related descendants of a single ancestral finch which had relatives on the South American mainland. Careful analysis showed that each of the 12 new species was confined and adapted to a specific habitat on a specific island. The finches, now known as “Darwin’s finches” (Figure A 12.24), clearly support both descent with modification and natural selection. Hawaiian honeycreepers (Figure B 12.24) are a more colorful but also more endangered example of the same evolutionary process of adaptive radiation. Bills ranging from thick and heavy (finch- like) for seed-eaters to long and curved for probing flowers illustrate the variations by which descendants of a single, original finch-like colonizer adapted to multiple ecological niches on the islands. Unfortunately, human destruction of habitat and introductions of rodents, the mongoose, and the mosquito which carries avian malaria have caused the extinction of 15 honeycreeper species, and still threaten the species which remain.

Altogether, the fossil record, homologies, analogies, vestigial structures, molecular unifor- mity and diversity, and biogeography provide powerful scientific evidence for the descent of today’s species from common ancestors. Some details of natural selection have been and are still being modified. However, the remarkable biological discoveries of the 150 years since Darwin published The Origin of the Species have dramatically strengthened support for his theory. Moreover, Darwin’s theory continues to enlighten new discoveries. Perhaps we could paraphrase Dobzhansky: Everything in biology makes sense in the light of evolution. The only piece still missing from the evidence puzzle is direct observation of the process itself. Darwin thought that humans could never witness evolution in action because of the vast time periods required. For once, however, he was mistaken; evolution in action is the subject of the next lesson.
Evolution is not “just a theory” as a scientific theory, it explains and unifies the entire field of biology and has a great deal of evidence supporting it.
The evidence includes the comparisons and observations Darwin included in his Origin,
and new knowledge from genetics and molecular biology, added since the Origin was published.
Darwin used the fossils known in his time as evidence for his ideas, and today’s record is even more convincing.
Often, fossil species first appear in older rocks, and disappear in younger rocks, pro- viding evidence that species change.
Changes in climate indicated by geology correlate with changes in fossil species and their adaptations, supporting the idea of natural selection.
The fossil record for horses shows gradual changes which correspond to changes in the environment.
Many basic similarities in comparative anatomy support recent common ancestry.
Similarities in structure for closely related species are homologous.
Similarities in structure among distantly related species are analogous if they evolved independently in similar environments. They provide good evidence for natural selec- tion.
Examples of evidence from embryology which supports common ancestry include the tail and gill slits present in all early vertebrate embryos.
Vestigial structures are reduced and perhaps even nonfunctional, but homologous to fully developed and functional similar structures are in a closely related species; these support the idea of natural selection.
www.ck12.org 586
Cavefish without sight or pigment and humans with goose bumps illustrate the concept of vestigiality.
The universality of DNA for genes, amino acids to build protein enzymes and the Genetic Code is strong evidence for common ancestry.
Similarities in metabolic pathways such as DNA replication and transcription and cellular respiration are further evidence for common ancestry.
Within these similarities are differences in the sequence of As, Ts, Cs, and Gs due to the accumulation of mutations.
Comparison of DNA sequences supports descent with modification and can be used to clarify evolutionary relationships.
A Cladogram is a tree-like diagram showing evolutionary relationships which can be construction from one or a number of kinds of comparison data; DNA sequence com- parisons are often used.
Darwin’s Theory of Evolution is strongly supported and also helps to explain many more recent discoveries, such Mendel’s work in genetics and the molecular biology of DNA and protein.
Changes in geographic features such as land bridges explain puzzling fossil species distributions.
Older fossil distributions suggest that the continents have joined and separated during Earth’s history.
Plate tectonics explain the distant locations of closely related species as the result of continental drifting.
Both Darwin and Wallace proposed that oceanic island chain species often descended from a single colonizing mainland species and adapted to open niches through natural selection.
Galapagos finches (Darwin’s finches) and Hawaiian honeycreepers each fill many dif- ferent ecological niches as the result of adaptive radiation from a single colonizing finch-like ancestor.
Why is it wrong to say that the Theory of Evolution is “just a theory”?
How did Darwin use the fossil record to support descent from common ancestors and natural selection?
Summarize how the fossil record for ancestors and relatives of the horse supports the relationship between evolution and changing environments.
Compare and contrast homologous and analogous structures as evidence for evolution.
Give two examples of evidence from embryology which support common ancestry.
Use an example to show how vestigial structures support evolution by natural selection.
List the molecular similarities found in all species of organisms, which support common ancestry.
Interpret the following cladogram in terms of evolutionary relationships and the DNA data which could have been used to construct it.
Relate the distribution of plants and animals to changes in geography and climate, using at least one specific example.
Use a specific example to illustrate the explanation given by both Darwin and Wallace for the distribution of few, closely related species across island chains.
David Quammen. 1997. The Song of the Dodo: Island Biogeography in an Age of Extinctions. Scribner.
Jonathan Weiner, The Beak of the Finch: A Story of Evolution in Our Time (Alfred
A. Knopf, 1994).
http://www.pbs.org/wgbh/evolution/library/01/6/l_016_01.html
absolute (radioisotope) dating A technique for dating fossils based on exponential de- cay of a radioactive isotope incorporated into the rock at the time of its formation or the fossil at the time of the organism’s death.
adaptive radiation A pattern of speciation which involves the relatively rapid evolution from a single species to several species to fill a diversity of available ecological niches.
analogous traits Similar structures with identical functions shared by distantly related species; analogous traits result from natural selection in similar environments, but they evolve independently.
biogeography The study of patterns of distribution of species on continents and islands.
cladogram A tree-like diagram showing evolutionary relationships according to a given set of data, such as molecular data.
www.ck12.org 588
comparative anatomy The study of the similarities and differences in organisms’ struc- tures.
comparative embryology The study of the similarities during the embryological devel- opment of vertebrate animals; reveals homologies which form during development but may later disappear.
embryology A branch of comparative anatomy which studies the development of verte- brate animals before birth or hatching.
fossil The mineralized remains of an animal, plant, or other organism.
fossil record An arrangement of all known fossils according to their position in time, using rock layer and radiometric dating.
homologous structures Structures which descended (evolved) from the same structure within a common ancestor; may or may not serve the same function.
homology Similarity which has resulted from shared ancestry.
hypothesis A proposed, testable answer to a question or explanation of an observation.
island biogeography The study of archipelagos (oceanic island chains) as isolated sites for evolution.
paleontology The study of fossils to explore the history of life.
relative dating A technique for aging fossils based on comparing their positions within rock layers; fossils in lower layers are usually older than fossils in upper layers.
theory An explanation which ties together or unifies a large group of observations.
vestigial structure Structures which are reduced and perhaps even nonfunctional in one species but homologous to functional structures in a closely related species.
Which type of evidence for evolution is most convincing to you?
Evidence confirms that evolution is a powerful theory. What other examples of the- ories have you encountered in your study of science? How would you compare their importance to the importance of evolution?
In this lesson, we have used the terms hypothesis, law, and theory. How would you explain the differences between these scientific ideas?
Recognize that the process of evolution by natural selection continues to change our world and our selves, both despite and because of our best efforts to control it.
Understand that we have added direct observation of natural selection to the evidence for evolution.
Evaluate the importance of artificial selection to human life.
Discuss our use of hybridization to improve yield and adapt crops to many climates.
Explain how cloning contradicts the principles of natural selection.
Compare genetic engineering to traditional methods of breeding and domestication.
Use the concept of natural selection to explain the resistance of bacteria to antibiotics and insects to pesticides.
Explain why an individual bacterium cannot on its own change from sensitive to re-
sistant towards antibiotics.
Assess the severity of the problem of antibiotic resistance.
Recognize that viral epidemics occur when chance viral mutations adapt the virus to new hosts.
Describe the evidence for natural selection among Darwin’s finches documented by the Grants.
Much of the immediate success of Darwin’s book was due to his careful comparison of his new idea of natural selection to the well-known breeding of animals. Darwin was especially interested in pigeons, and his observations of their many varieties inspired his own early thinking. Humans have relied on artificial selection ever since we first put seeds in the ground some ten thousand years ago. Today, our continuing efforts to develop crops and animals for food, work, and companions have expanded beyond breeding to include genetic engineering. Dismay about our effects on the environment is encouraging us to see ourselves more as a part of nature than above it; perhaps we will eventually abandon Darwin’s term “artificial selection” in favor of coevolution. Evolution by natural selection is not just an explanation of the history of life. The process of Darwin’s theory clearly continues, changing our world and ourselves - both despite and because of our best efforts to control it. And we have reached beyond Darwin’s wildest expectations; we now have direct observations of natural selection to add to the overwhelming evidence for evolution.
www.ck12.org 590
The range of variations induced in relatively short periods of time by animal breeders con- vinced Darwin that natural selection across geologic time could have produced the great diversity of present life. Domestication of animals has resulted in the remarkable variety of dogs (Figure 12.25) from wolves, as well as cattle, horses, llamas, camels, and a few evolutionary dead-ends, such as the donkey.

However, artificial selection has resulted in the achievement that extends far beyond our immediate, intentional goals. Our initial cultivation of plants such as corn (Figure 12.26) played a role in the eventual development of human civilization.
Since Darwin’s time, selective breeding and hybridization – mixing of separate species - has become even more sophisticated. We have further hybridized high-yield hybrids with local varieties throughout the world, intentionally adapting them to local climates and pests. Unfortunately, our widespread destruction of habitat is eroding the species and genetic diversity which provides the raw material for such efforts. Moreover, against our intent, our hybrids sometimes interbreed with natural varieties in the wild, leading to what some call genetic pollution. An example is a tiger, thought to be pure Bengal but actually a Bengal-Siberian hybrid, released in India to demonstrate the survival abilities of captive- raised tigers. The tiger did survive – to pollute the genetically pure Bengal population in a national park with northern-adapted Siberian genes (Figure 12.27).
The new field of biotechnology has dramatically changed our quest to improve upon natural selection. Ironically, one new development intentionally undermines the very foundation of Darwin’s theory. As the first mammal to be cloned, a sheep name Dolly showed breeders of animals from farms to racetracks that they could copy “ideal” individuals without the bothersome variation which accompanies sexual reproduction (Figure 12.28). Many people hope that future decisions about cloning will consider Darwin’s lessons about the value of variation in unpredictable, changing environments.


Figure 12.27: The natural genes which adapted the Indian Bengal tiger (Panthera tigris tigris, left) and the Russian Siberian tiger (Panthera tigris altaica, right) to their unique habitats were mixed or “polluted” when a captive hybrid was released into a national park in India. The “escape” of non-native genes into a wild population is genetic pollution. (4)
www.ck12.org 592

Another contribution of biotechnology is genetic engineering, the transfer of a gene from one organism to another. First, we inserted the human gene for insulin into bacteria, which – as bacteria use the same universal Genetic Code as we use – read the DNA and produced the human protein for use by diabetics. Many more cost-saving and designer medical advances have followed, including
production of clotting factors for hemophiliacs
vaccines for devastating diseases such as hepatitis B
a breast cancer “designer drug,” herceptin
the potential for cheap, effective vaccines in fruits such as bananas
We have extended genetic engineering to agriculture, improving range, nutrition, resistance to disease, and other aspects of life. Transgenic animals - which possess genes from another species - now produce vaccines and hormones, serve in scientific research, and entertain us as pets (Figure 12.29). However, as for traditional agriculture, fears surround potential cross-pollination and interbreeding with wild populations. Modified genes have been found in plants up to 21 km (13 miles) away from their source. If such transfers spread resistance to herbicides or pesticides to wild populations, they will have defeated their intended purpose.
In his book, The Botany of Desire, Michael Pollan questions our feelings of superiority over our domesticated plants and animals. Discussing our domestication of the apple for its sugar,

www.ck12.org 594
the tulip for its beauty, marijuana for its psychogenic effects, and the potato for its food value, Pollan takes the plants’ view of the evolving relationships. Could it not be that, as we have selected and modified these plants, they have also selected us for our powers to ensure their survival and reproduction – and changed us in the process? Are domestication of animals, cultivation of plants, and selective breeding actually forms of coevolution? Pollan’s delightful yet sobering treatise may reflect a growing realization that we humans are as much a part of nature as any other species. Yes, we can influence evolution in a number of ways. However, we remain subject to natural selection, and every choice we make has effects on evolution – including our own. As we have already seen, and will see again in the next topic, our choices often have unintended effects.
In almost unprecedented actions during May 2007, United States government agencies put a US citizen on a no-fly list, urged border agents to detain him, failed to detect his re-entry into the US, and eventually ordered him into involuntary isolation, urging individuals who had flown with him on several international flights to be tested for XDR-TB. Why were such drastic measures needed? What is XDR-TB, and how did it originate? The answers show evolution in action today - in a way that all of us need to understand for our own well-being.
Tuberculosis (TB) has infected and killed humans since at least 4000 BCE. Today, over one-
third of the world’s population has been exposed to the bacterium which causes tuberculosis (Figure 12.30), but 90% of those carry the microorganism without symptoms. In the past, the 10% who did develop the characteristic lung infection had a 50% chance of dying. The advent of antibiotics in the mid-20th century dramatically improved survival, although the slow-growing bacteria required treatments of 6-12 months rather than days. Just 40 years later, in the 1990s, a new strain appeared with a mortality rate comparable to lung cancer
up to 80%. MDR-TB, or multi-drug resistant TB, is not treatable by two of the most effective anti-TB antibiotics. Then, about the year 2000, a second, more menacing strain emerged. XDR-TB, or extensively drug-resistant TB, is not treatable by either the two major drugs or the less-effective “second line” drugs now used to treat MDR-TB. Late in 2006, an epidemic of XDR-TB developed in South Africa. Currently there are no available drugs that can effectively treat this strain of TB.
Clearly these strains of TB are new, and changing rapidly. The evolution of resistance is a growing problem for many disease-causing bacteria and also for parasites, viruses, fungi, and cancer cells. The “miracle” of drug treatment which appeared to protect humans from disease may be short-lived. How does resistance happen? How can we prevent it?
First, recognize that resistance describes the bacterium (or other microorganism) – not the human. Bacteria multiply much more rapidly than humans, and therefore can evolve much more rapidly. Consider a population of bacteria infecting an individual with tuberculosis. Like all populations, individuals within that population show variation. Mutations add more variation. By chance, mutation may change the chemistry of one or a few bacteria so that they are not affected by a particular antibiotic. If the infected human begins to take antibiotics, they change the environment for the bacteria, killing most of them. However, the few bacteria which by chance have genes for resistance will survive this change in environ- ment - and reproduce offspring which also carry the genes. More and more of the bacterial population will be resistant to antibiotics, because the antibiotics select for resistance. The bacteria are merely evolving in response to changes in their habitats! If the resistant bacteria are transmitted to another human “habitat,” their population continues to expand, and if the new “habitat” takes different drugs, natural selection may result in multi-drug resistance (Figure 12.31).
How widespread is the problem? Staphylococcus aureus bacteria first showed resistance to penicillin just four years after the drug was put into use; today, some strains have shown resistance to nearly all antibiotics. These are now known as one of several “superbugs.” The Human Immunodeficiency Virus (HIV) has become resistant to several antiviral drugs, and cancer cells within an individual often evolve resistance to chemotherapy drugs. Pesticide resistance is evolving in a similar manner; U.S. crop losses to insect pests have increased from 7% in the 1940s to more than 13% in the 1980s, despite the use of more types of pesticides in the 1980s.
What can we do about this particular instance of evolution which we have unwittingly encouraged? In general, we should reduce the use of antibiotics where possible and safe in order to lessen the selective pressure on bacteria. Here are some practices to keep in mind:
www.ck12.org 596
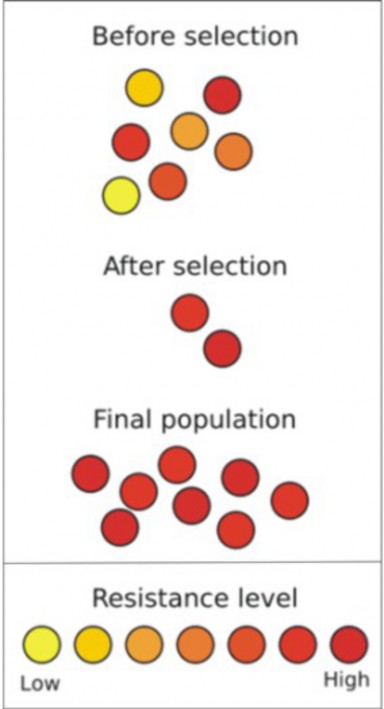
Figure 12.31: The development of resistance to antibiotics is a classic example of natural selection. Before selection, a number of heritable variations in level of resistance exist within the population (see legend at bottom). After selection by antibiotics, only those bacteria resistant to antibiotics survive. Only these resistant bacteria reproduce, so that the final population contains a greater proportion of resistant bacteria. (25)
Don’t take antibiotics for viral infections such as colds and flu; they act only on bacteria.
When antibiotics are appropriate, take them exactly as prescribed, and complete the entire course.
Never take antibiotics which are left over from an earlier illness or prescribed for some- one else.
Consider purchasing meats and other animal products from animals not treated with antibiotics.
Consider purchasing organic produce, which is not treated with pesticides.
Resist the use of pesticides in your own gardens.
We have unintentionally sped up the evolution of microorganisms, but at the same time, their development of resistance has given us a window into the process which underlies all changes in life, natural selection.
Much more passively and with a clear understanding of our lack of control, humans have watched viruses rapidly evolve through mutation to cause frightening worldwide epidemics, or pandemics - from the 1918 “Spanish flu” through Severe Acute Respiratory Syndrome (SARS) and West Nile virus, to the widely anticipated “avian flu” caused by a highly pathogenic viral subtype of influenza A (Figure 12.32), known as H5N1, and the 2009 ”swine flu” caused by the H1N1 influenza virus. Figure 8 shows the increase in human in- fections and deaths from H5N1. Mutations have adapted it for life in birds and in humans, and for transmission from bird to bird and bird to human. If a future mutation adapts it for effective transmission from human to human, a serious epidemic could result. If, as some argue, influenza pandemics occur in cycles, we are overdue for a dramatic demonstration of evolution and natural selection.
Peppered moths (Figure 12.33) are mostly white with black specks – a color pattern which hid them for centuries from predatory birds as they rest against lichen covered tree trunks. However, soot from the Industrial Revolution darkened the trees and destroyed their cam- ouflage, selecting instead for the dark mutants which occasionally appeared. Gradually the population shifted to a dark color – an instance of natural selection that was directly observed by Englishmen of the time. Subsequent improvements in air pollution control have cleaned up the environment, and the English now note a new change: the trees have lightened, and moth populations are returning to their original coloration. These direct observations of natural selection would have delighted Darwin (except perhaps for the pollution) just a few years earlier.
Much more intentionally, biologists Peter and Rosemary Grant have devoted more than 30 years to a study of two species of Darwin’s finches on one of the Galapagos islands (Figure 12.34). Catching, weighing, and recording the seed species eaten by hundreds of these birds, they have witnessed changes in beak size which clearly correlate with changes in weather
www.ck12.org 598
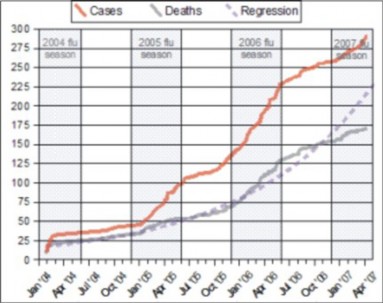

www.ck12.org 600
and availability of food. A severe drought and food shortage in 1977 led to a significant change. Birds whose small beaks could not crack the tough remaining seeds died, and the larger-beaked individuals who survived reproduced. The following year, offspring were larger bodied and larger-beaked, showing that natural selection led to evolution. A rainy winter in 1984-1985 reversed the trend; more soft seeds were produced, and the smaller beaked finches survived and reproduced in greater numbers than their large-beaked cousins.

Jonathan Winter eloquently describes the Grants’ work and discoveries in his Pulitzer Prize- winning The Beak of the Finch, A story of Evolution in our Time. His words urging that we see evolution as ongoing for all life make a fitting conclusion to this lesson and chapter:
(Source: http://en.wikiquote.org/wiki/Beak_of_the_Finch)
The process of evolution by natural selection continues to change our world and our selves, both despite and because of our best efforts to control it.
Beyond Darwin’s expectations, we have added direct observation of natural selection to the overwhelming evidence for evolution.
Humans have designed and produced crops, work animals, and companions through artificial selection.
Cultivation of crops gave us the freedom to develop civilization.
Hybridization improves the yield of crop species and adapts them to various environ- ments.
Habitat destruction is destroying raw materials for hybridization, and “escape” of “artificial” genes is “polluting” wild species.
Cloning has the potential to reproduce exact copies of selected individuals, but it goes against the principles which govern natural selection.
Genetic engineering, like traditional methods of breeding and domestication, designs medicines, plants, and animals to suit our goals.
Unlike traditional breeding, genetic engineering chooses single genes and can transfer them from one species to another completely unrelated species – making it faster, more precise, and far more powerful.
In both GE and traditional breeding, the potential for genetic pollution remains. Pol- lution is probably more likely for genetic engineering because developments proceed so quickly.
Products of genetic engineering include insulin and growth hormone, vaccines in milk and bananas, produce with longer growing season and shelf life and more nutrition.
Michael Pollan suggests that we are coevolving with our domesticated crops, animals, and pets, rather than producing them – in other words, that our products are domes- ticating us as we domesticate them!
Bacteria have developed serious levels of resistance to antibiotics because humans have introduced a new selective force into their environments (our bodies).
An individual bacterium has its own set of genes. If these genes do not confer resistance to antibiotics, the bacterium by itself cannot develop resistance. A population can develop resistance if some of its members have, by chance, the gene for resistance.
The evolution of antibiotic resistance has already resulted in a number of bacteria resistant to most known antibiotics; these are sometime called “superbugs.”
Actions you can take to prevent or slow the evolution of antibiotic resistance include:
Don’t take antibiotics for viral infections.
Take prescribed antibiotics exactly as prescribed.
Never take antibiotics which are left over or belong to someone else.
Consider purchasing meats from animals not treated with antibiotics. www.ck12.org 602
Consider purchasing organic produce.
Resist the use of pesticides in your own gardens.
Viral epidemics occur when chance viral mutations adapt the virus to new hosts or new methods of transmission.
Peppered moth populations changed color as the Industrial revolution changed the color of their habitat.
Peter and Rosemary Grant studied two closely related species of Darwin’s finches and recorded changes in beak size and body size which paralleled changes in weather.
List the ways in which we have directly observed evidence for evolution and/or natural selection.
Describe the importance of artificial selection to human life.
What is genetic pollution and why does it matter?
Compare cloning to natural selection.
Give examples of useful products of genetic engineering.
Explain Michael Pollan’s ideas about our relationship with our domesticated crops, animals, and pets, and give your opinion about them, using examples from your own experience.
Use the concept of natural selection to explain the resistance of bacteria to antibiotics and insects to pesticides.
Explain why an individual bacterium cannot on its own change from sensitive to re-
sistant to antibiotics.
Choose two actions you think would be most likely to control the increase in antibiotic resistance, and support your choices with examples from your own experience.
In what way do viral epidemics demonstrate evolution?
Michael Pollan, 2001. The Botany of Desire, Random House, 2002.
David Quammen, 1997. The Song of the Dodo: Island Biogeography in an Age of Extinctions. Scribner.
Carl Sagan, 1980. Cosmos. Random House New Edition, May 7, 2002, 384 pgs – also available in video and DVD, as Cosmos: A Personal Voyage.
Jonathan Weiner, 1994. The Beak of the Finch: A Story of Evolution in Our Time.
Alfred A. Knopf.
http://www.biologycorner.com/worksheets/peppermoth_paper.html
http://crustacea.nhm.org/people/martin/publications/pdf/103.pdf
artificial selection Animal or plant breeding; artificially choosing which individuals will reproduce according to desirable traits.
cloning The process of creating an identical copy of an organism.
coevolution A pattern in which species influence each other’s evolution and therefore evolve in tandem.
genetically modified organism (GMO) An organism whose genes have been altered by genetic engineering.
genetic engineering The manipulation of an organism’s genes, usually involving the in- sertion of a gene or genes from one organism into another.
genetic pollution The natural hybridization or mixing of genes of a wild population with a domestic or feral population.
geologic time Time on the scale of the history of Earth, which spans 4.6 billion years.
mutation A change in the nucleotide sequence of DNA or RNA.
natural selection The process by which a certain trait becomes more common within a population, including heritable variation, overproduction of offspring, and differential survival and reproduction.
transgenic animal An animal which possesses genes of another species due to genetic engineering.
www.ck12.org 604
To what extent do you think that humans have removed themselves from natural selection?
In what ways do you still feel subject to “natural” selective pressures?
How effective do you think the measures to limit evolution of antibiotic resistance will be? Are you willing to support them?
Do you think the benefits of genetic engineering outweigh the risks? Are there certain products you support, and others you oppose? Which ones, and why?
//upload.wikimedia.org/wikipedia/commons/b/b1/Wallace_biogeography.jpg. Public Domain.
http://en.wikipedia.org/wiki/Image:Horseevolution.png. GNU-FDL.
George. http://commons.wikimedia.org/wiki/Image: Charles_Darwin_by_G._Richmond.jpg. Creative Commons.
http://commons.wikimedia.org/wiki/Image:Siberian_Tiger_sf.jpg. CC-BY-SA-2.5, CC-BY-SA-2.5, 2.0, 1.0.
John Romanes. http://commons.wikimedia.org/wiki/Image:Homology.jpg. Public Domain.
http://commons.wikimedia.org/wiki/Image: Blaufu%C3%9Ft%C3%B6lpel_%28Sula_nebouxii_excisa%293.jpg. GNU-FDL, CC-BY-SA-2.0.
http://commons.wikimedia.org/wiki/Image:Atoll_forming-i18.png. Public Domain.
Romanes’s copy of Ernst Haeckel’s drawings. http://commons.wikimedia.org/wiki/Image:Haeckel_drawings.jpg. Public Domain.
Joanne and Matt. http://www.flickr.com/photos/joanne_matt/127758696/. CC-BY.
http://commons.wikimedia.org/wiki/Image:Zebrafisch.jpg. Copyright Free, Copyright Free.
http://commons.wikimedia.org/wiki/Image:Canis_lupus_lycaon_03.jpg. GNU-FDL, CC-BY-SA-2.5.
http://commons.wikimedia.org/wiki/Image:Palila.jpg http://en.wikipedia.org/wiki/Image:Iiwi.jpg http://commons.wikimedia.org/wiki/Image:Amakihi.jpg. Public Domain, Public Domain, Public Domain, Public Domain.
Jerry Crimson Mann. http://en.wikipedia.org/wiki/Image:Evolution_pl.png. Creative Commons.
http://commons.wikimedia.org http://commons.wikimedia.org/wiki/Image:Drosera_rotundifolia_ chromolithograph.jpg/wiki/Image:Calypso_bulbosa_Nordens_Flora_416.jpg. Public Domain.
http://commons.wikimedia.org/wiki/Image:Bactrian.camel.sideon.arp.jpg http://commons.wikimedia.org/wiki/Image:Guanaco_09.24.jpg. GNU-FDL, GNU-FDL, Public Domain, Public Domain.
London Sketchbook. http://commons.wikimedia.org/wiki/Image:Caricatura_de_Darwin.jpg. Public Domain.
Owen Stanley. http://commons.wikimedia.org/wiki/Image:HMSBeagle.jpg. Public Domain.
http://en.wikipedia.org/wiki/Image:
Snider-Pellegrini_Wegener_fossil_map.gif. Public Domain.
John Doebley. http://commons.wikimedia.org/wiki/Image:Teosinte.png. CC-BY-2.5.
http://commons.wikimedia.org/wiki/Image:Geochelone_nigra.png. CC-BY-2.5.
http://commons.wikimedia.org/wiki/Image:Amblyopsis_spelaeus.jpg http://en.wikipedia.org/wiki/Image:Crane_fly_halteres.jpg http://commons.wikimedia.org/wiki/Image:Cold_urticaria.jpg. (d)Public Domain.
Mark Putney. http://www.flickr.com/photos/putneymark/1226674080/. CC-BY-SA.
Cambridge University Press. http://commons.wikimedia.org/wiki/Image:Fuegian_BeagleVoyage.jpg. Public Domain.
www.ck12.org 606
http://commons.wikimedia.org/wiki/Image:Pfautaube.jpg http://commons.wikimedia.org/wiki/Image: Fiary_Swallow_%28Wing_Pigeon%29.jpg. GNU-FDL.
http://commons.wikimedia.org/wiki/Image:Antibiotic_resistance.svg. Public Domain.
Elembis. http://commons.wikimedia.org/wiki/Image: Mutation_and_selection_diagram.svg. GFDL.
Kipala. http://commons.wikimedia.org/wiki/Image:Voyage_of_the_Beagle.jpg. GFDL.
http://commons.wikimedia.org/wiki/Image: Biston.betularia.f.carbonaria.7209.jpg. CC-BY-SA-2.5, CC-BY-SA-2.5.
http://commons.wikimedia.org/wiki/Image:Lightmatter_chimp.jpg http://commons.wikimedia.org/wiki/File:Male_silverback_Gorilla.JPG http://commons.wikimedia.org/wiki/Image:Papio_ursinus.jpg. GNU-FDL, CC-BY-2.5, GNU-FDL, CC-BY-SA-2.5.
Waitak - - Duy Đức. http://en.wikipedia.org/wiki/Image: H5n1_spread_%28with_regression%29.png. GNU-FDL.
Mariana Ruiz Villarreal. http://commons.wikimedia.org/wiki/File: Megatherium_americanum_complete.JPG. Public Domain.
Janice Carr. http://commons.wikimedia.org/wiki/Image: Mycobacterium_tuberculosis_8438_lores.jpg. Public Domain.
Bev Sykes. http://commons.wikimedia.org/wiki/Image:Seattle-giraffe3.jpg. CC-BY-2.0.
Charles Darwin, Alexei Kouprianov. http://commons.wikimedia.org/wiki/Image:Darwin_divergence.jpg. Public Domain.
www.ck12.org 608
Analyze the relationship between Darwin’s work and Mendel’s discoveries.
Explain the goal of population genetics.
Describe the relationship between genes and traits.
Differentiate between genes and alleles.
Connect alleles to variations in traits.
Distinguish environmental effects on gene expression from allelic variations in genes.
Describe the relationship between mutations and alleles.
Explain the causes and random nature of mutation.
Compare rates of mutation in microorganisms to those in multicellular organisms.
Analyze the ways in which sexual reproduction increases variation.
Relate mutation and sexual reproduction to natural selection.
Explain why populations, but not individuals, can evolve.
Define a population’s gene pool.
Distinguish between a population’s gene pool and a gene pool for a single gene.
Analyze the usefulness of the gene pool concept.
Explain how to determine allele frequencies.
Define evolution in terms of allele frequencies.
Discuss what is meant by a population which is fixed for a certain gene.
Show how allele frequencies measure diversity.
Evaluate the significance of a change in allele frequency.
If you have ever taken something apart to find out how it works, you will understand biol- ogists’ delight in exploring, since Darwin’s findings, how evolution actually works. Darwin gave us the keys to unlocking this mystery by describing natural selection and common an- cestry as a scientific explanation for the similarities and differences among the millions of Earth’s species – living and extinct. However, his theory depended on the ideas that traits could vary, and that variations were heritable – and even Darwin was puzzled as to how this might work. Another biological giant, Gregor Mendel, was a contemporary of Darwin’s, but his now-famous work with pea plant inheritance was not widely known or appreciated until after both scientists had died. During the 20th century, “re-discovery” of Mendel’s work stimulated extensive research in genetics, and the identification of DNA as the universal genetic material of life, brought “heritable variation” into sharp focus. A branch of the new field of Population Biology (discussed in the Populations Chapter) finally combined what Mendel and Darwin had begun separately - the exploration of population genetics and the evolution of populations.
You have studied genetics, DNA, and Darwinian evolutionary theory in previous chapters. You have had the opportunity to learn far more about biology than either Darwin or Mendel could imagine. You are prepared, then, to join biologists in exploring how evolution works. You know that biologists have massive amounts of evidence that natural selection and evolution happen. This chapter will explore what we know about how molecules, genes, and populations change – with the ultimate goal of understanding how the “Origin of Species” produced the vast diversity of life on Earth.
As you’ve learned, each cell of an organism contains all the information needed to build the entire organism in the DNA of its chromosomes (Figure 13.1). A gene is a segment of DNA which has the information to code for a protein (or RNA molecule) (Figure 13.2). For many genes, the protein product controls or at least contributes to a particular trait. For example, enzymes are proteins which are important in the biochemical reactions controlling many cellular processes.
For example, the gene for the enzyme tyrosinase controls the chemical reaction which makes melanin, a brown-black pigment which colors the skin and hair of many animals, including humans. The gene is the “recipe” for tyrosinase; tyrosinase is the protein, and the trait is coloring of the skin or fur. The rabbit in Figure 13.3 has brown fur because its DNA contains a gene for tyrosinase.
www.ck12.org 610
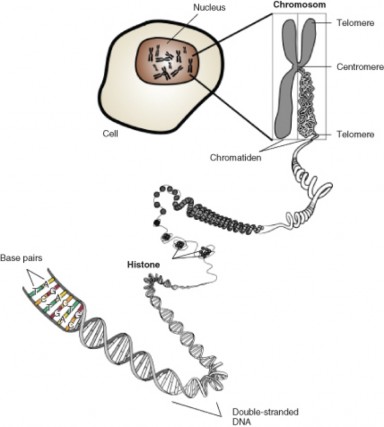
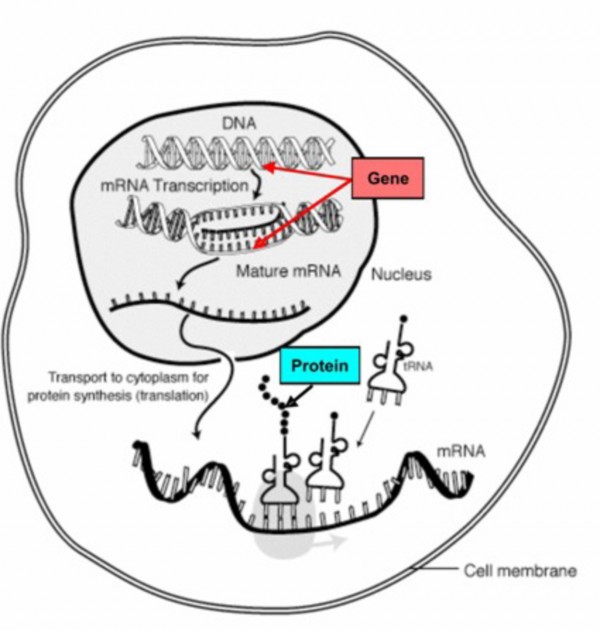
www.ck12.org 612

Genes often have different forms – slightly different nucleotide sequences – known as alleles. In some rabbits, an alternative form of the gene for fur color makes non-functional tyrosinase. A change in the sequence of As, Ts, Cs, and Gs changes the sequence of amino acids in the protein and alters or destroys its activity. This allele is recessive, and can cause lack of pigment – an albino rabbit (Figure 13.4). We will refer to the dominant gene for brown fur as B, and the recessive gene for albinism as b.

Recall that Mendel showed that humans and rabbits have two copies of each gene, or ”herita-
ble unit” – one from each of our parents. The two alleles we received make up our genotype. For simple Mendelian traits, our genotype determines our physical appearance, or pheno- type, for that trait. A rabbit having two copies of the mutant, recessive gene cannot make tyrosinase – or melanin; its genotype is bb, and its phenotype is albino. A rabbit having either one or two copies of the gene for tyrosinase makes melanin; its genotype is either Bb or BB, and in either case, because B is dominant, its phenotype is brown. Brown rabbits which have different alleles (genotype Bb) are heterozygous, and brown rabbits with identical alleles (BB) are homozygous. Albino rabbits with identical recessive alleles (bb) are also homozygous.
Keep in mind that the environment can influence the expression of a gene – its transcription and translation to produce a protein. By preventing or promoting the expression of certain genes, the environment can cause variation in traits, but note that this influence is not at all goal-directed and does not result in heritable change. If you do not water or fertilize your garden, the plants will be short and stunted, but their small size is not a heritable change. Let’s return to rabbits to see how this kind of variation happens at the level of genes and molecules.
A third allele for rabbit fur coloration codes for a form of tyrosinase which is temperature- sensitive. At low temperatures, the enzyme makes melanin normally. However, higher temperatures denature the enzyme in the same way that cooking changes egg white; at higher temperatures, the enzyme is misshapen and cannot make melanin. The rabbit in Figure
is homozygous for temperature-sensitive tyrosinase. The main part of its body, which has a higher temperature, is white because its tyrosinase does not work at high temperatures. The tips of the rabbit’s ears, nose, and feet, however, are black, because the enzyme can work at the slightly lower temperatures of these extremities. What do you think such a rabbit would look like if it developed in the arctic? The tropics?
In conclusion, heritable variations, which Darwin knew were necessary for natural selection, are determined by the variety of alleles, or different forms of genes, which build proteins. The environment may add to the variety of phenotypes by influencing their expression, but the genes themselves remain unchanged. Only genes and their alleles can be sources of heritable variations in traits.
Genetic variation results from the diversity of alleles. How do alleles form?
You may recall that mutations change the sequence of nucleotides in DNA. Mutations can alter single nucleotides or entire chromosomes Figure 13.6, and they are the sole source of new alleles.
Even a single nucleotide substitution can cause major changes in an organism; Figure 13.7 shows the molecular and cellular effects of the well-known substitution mutation which causes
www.ck12.org 614

sickle-cell anemia in humans.
Caused by ultraviolet or ionizing radiation, certain chemicals, or viruses, mutations occur entirely by chance; they are not in any sense goal-directed. Usually, they reduce an organ- ism’s fitness - its ability to survive and reproduce. However, many mutations are neutral; they have no effect on an organism’s fitness. A few may actually improve fitness. Sickle-cell hemoglobin (Hemoglobin S – see Figure 13.7) prevents malarial infection, so in equato- rial environments where malaria is prevalent, one copy of the Hemoglobin-S allele increases survival and reproduction.
New alleles arise only by chance mutations, yet without them, there would be no diversity and no evolution. Although they are rare due to repair mechanisms, mutations provide the creative potential for adaptation to environmental change.
Rates of mutation depend largely on reproductive rate, and are highest in bacteria and viruses. HIV, for example, can produce over one trillion new viruses per day, and each replication of its genome provides an opportunity for mutation. Their high mutation rates explain why viruses and bacteria so often become resistant to drug treatments.
Mutations resulting in heritable variation for multicellular organisms happen constantly, but at a lower rate. In part, this is because mutations in body cells do not affect the DNA in eggs and sperm. While this prevents many damaging mutations from dooming offspring, it also reduces diversity and the potential for adaptation to changing environments. Sexual reproduction, however, compensates at least in part for this loss.
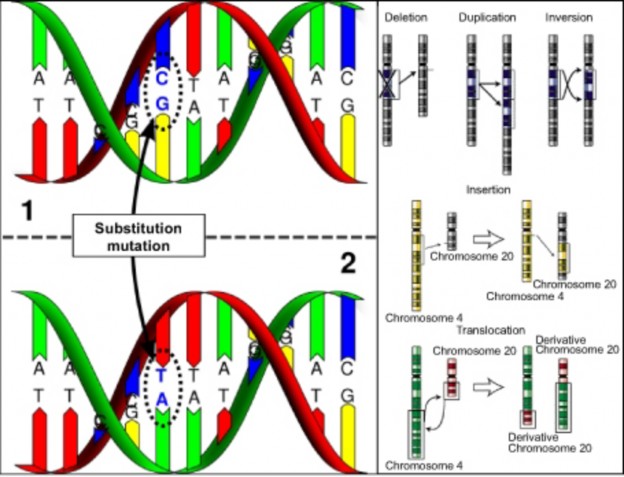
as in chromosomal mutations (B). (9)
www.ck12.org 616
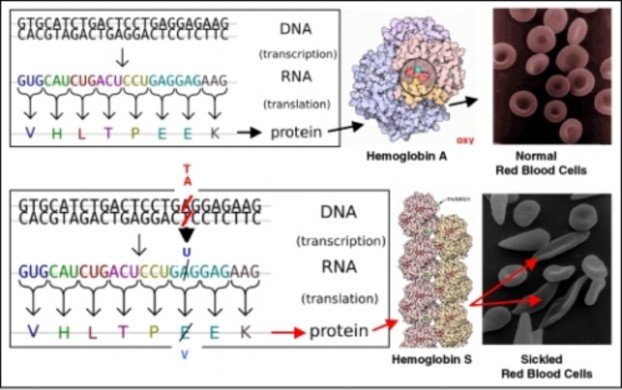

Although sexual reproduction cannot produce new alleles, meiosis and fertilization shuffle alleles from past mutations into new combinations Figure 13.8. As Mendel demonstrated, genes (which he called “factors”) segregate and sort independently during the formation of eggs and sperm (return to the Mendelian Genetics if needed), and fertilization is random. The result is that – by chance – each offspring has a unique combination of alleles – a tremendous source of variation and raw material for natural selection.
Individuals do not evolve. Natural selection may affect an individual’s chance to survive and reproduce, but it cannot change the individual’s genes. However, a population – a group of organisms of a single species in a certain area – evolves when natural selection imposes differential survival on individuals within it. Population genetics studies populations at the level of genes and alleles in order to discover how evolution works.
If we consider all the alleles of all the genes of all the individuals within a population, we have defined the gene pool for that population. Gene pools contain all the genetic variation
that raw material for natural selection – within a population. The gene pool for a rabbit population, for example, includes alleles which determine coat color, ear size, whisker length, tail shape, and more (Table 13.1). If a population geneticist wants to focus on the variation in an individual gene, she/he may look at the gene pool of all the alleles for that gene alone.
Consider a population of 100 rabbits, including 10 albinos and 90 brown rabbits. The proportion of each phenotype is a measure of variation in this population, but it does not show the true genetic diversity. The gene pool does. Two populations of 10 white and 90
www.ck12.org 618
brown rabbits – with identical phenotypic diversity - could differ, because brown rabbits could be either homozygous or heterozygous.
Rabbits, like humans, are diploid. Therefore, each population’s gene pool for coat color contains 200 alleles. The 10 albinos in each population contribute a total of 20 b alleles to each gene pool. However, if population #1 has 40 heterozygotes and population #2 just 20, their gene pools differ significantly.
In population #1, 50 homozygous dominant individuals contribute 100 B alleles, and the 40 heterozygotes contribute 40 more. The heterozygotes also contribute 40 albino b alleles. Thus, population #1 contains a total of 140 B alleles and 60 b alleles.
Population #2, on the other hand, has 70 homozygous dominant rabbits, which add 140 B alleles to the 20 from the heterozygotes – a total of 160 B alleles. Only 40 b alleles are present in the gene pool, so genetic diversity is lower. The use of gene pools allows population geneticists to see variation and the influence of natural selection in detail.
Table 13.1: Table of Phenotypes and Genotypes in Two Rabbit Populations
![]()
Phenotypes Number of
each phenotype
Genotypes Number of
each genotype
Alleles con- tributed
to gene pool
Total gene pool
![]()
Rabbit Pop- ulation #1
90 BB 50 100B 140B


90 Bb 40 40B + 40b

10 bb 10− 20b 60b
Phenotypes Number of
each phenotype
Table 13.1: (continued)
![]()
Genotypes Number of
each genotype
Alleles con- tributed
to gene pool
Total gene pool
![]()
Rabbit Pop- ulation #2
90 BB 70 140B 160B


90 Bb 20 20B + 20b

10 bb 10− 20b 40b
![]()
As Darwin saw, populations are the units of evolution. As Mendel showed but Darwin could not have seen, heritable units, which we now know as alleles, determine the amount of variation within a population. A measure of this variation is the population’s gene pool. Once again, individuals cannot evolve, but it is the individual organism that adapts to its environment. It is the gradual accumulation of a series of adaptations - through genetic change - in a lineage from the population that is evolution.
Our analysis of the rabbit populations (Table 13.1) showed that the relative numbers of various alleles are the best measure of variability in a gene pool (or a population). Population geneticists calculate the proportions, or frequencies, of each allele in order to study changes in populations. In fact, a change in allele frequency is the most precise measure of the process of evolution.
An allele’s frequency is the fraction (expressed as a decimal) of a population’s gene pool www.ck12.org 620
made up of that particular allele. In rabbit population #1 above, the gene pool for coat color included 200 alleles, 140 of which were B. The frequency of allele B for population #1 is 140/200 = 0.7. Because frequencies must total 1.0, and the only other allele is b, the frequency of allele b is 0.3. (Alternatively, we can calculate the frequency of allele b by dividing the number of this allele by the total number of alleles: 60/200 is again 0.3.) In population #2, 160 of 200 alleles are B, so the frequency of allele B is 0.8, and the frequency of allele b is 0.2.
If all the members of a rabbit population (#3) are homozygous brown, the frequency of B is 1.0, the frequency of b is 0, and there is no diversity. Allele B is said to be fixed, and until a mutation occurs, no possibility for evolution (with respect to this particular gene) exists. A population with frequencies of 0.5 for B and 0.5 for b would have maximum diversity for this two-gene system.
Any change in those frequencies across generations would reflect evolution of the population
the subject of the next lesson.
Population genetics studies populations at the level of genes and alleles in order to discover how evolution works.
Genes - segments of DNA - determine traits by coding for enzymes that control chemical reactions.
Many genes have several different forms known as alleles.
Alleles are responsible for the variation in traits.
The environment can affect the expression of some genes, but variations induced by the environment are not heritable.
Mutations – changes in single nucleotides or entire chromosomes - are the sole source of new alleles.
Ultraviolet or ionizing radiation, chemicals, or viruses cause mutations - in an entirely random way.
Rates of mutation are high in rapidly reproducing viruses and bacteria, allowing them to adapt quickly to changing environments. An example is drug resistance.
Rates of heritable mutations are lower in multicellular organisms because only germ cell mutations are passed on to offspring.
Sexual reproduction recombines existing alleles through meiosis and random fertiliza- tion, so that each offspring is unique.
Mutations and sexual reproduction provide the variety which is the raw material for natural selection.
Individuals experience natural selection, but not the genetic changes of evolution.
Populations evolve through genetic change.
A population’s gene pool includes all the alleles of all the genes of all the individuals within it.
A gene pool for a single gene includes all the alleles of that gene present in all individ- uals.
Analysis of a gene pool can reveal variation which is not visible in phenotypes.
Changes in allele frequency measure the process of evolution.
Allele frequency is the decimal fraction of a population’s gene pool made up of that particular allele.
A gene pool containing only one type of allele is fixed; it lacks variation and potential for evolution, at least until mutation occurs.
For a gene with two alleles, allele frequencies of 0.5 indicate maximum variation.
Any change in allele frequency reflects evolution of a population.
Describe the relationship between genes and traits.
Explain the goal of population genetics.
Differentiate between genes and alleles.
Distinguish environmental effects on gene expression from allelic variations in genes.
Describe the relationship between mutations, alleles, variation, natural selection, and chance.
Compare rates of mutation in microorganisms to those in multicellular organisms.
Explain why populations, but not individuals, can evolve.
Distinguish between a population’s gene pool and a gene pool for a single gene.
Explain how to determine allele frequencies.
Evaluate phenotype, genotype, and allele frequencies as measures of variation, as raw material for evolution, and as a measure of evolution.
http://chroma.gs.washington.edu/outreach/genetics/sickle/sickle-bean.html
allele An alternative form or different version of a gene.
allele frequency The fraction (usually expressed as a decimal) of a population’s gene pool made up of a particular allele.
evolution A change in the frequency of an allele (or genetic makeup) in a population. www.ck12.org 622
fitness The ability of an organism with a certain genotype to survive and reproduce, often measured as the proportion of that organism’s genes in all of the next generation’s genes.
gene A segment of DNA which codes for a protein or RNA molecule; a unit of inheritance.
gene pool Within a population, the sum of all the alleles of all the genes of all the indi- viduals.
genotype The genetic makeup of an organism; specifically, the two alleles present. heterozygous Describes a genotype or individual having two different alleles for a gene. homozygous Describes a genotype or individual having two copies of the same allele for
a gene.
mutation A change in the nucleotide sequence of DNA or RNA.
natural selection The process by which a certain trait becomes more common within a population, including heritable variation, overproduction of offspring, and differential survival and reproduction.
phenotype The physical appearance of an organism determined by a particular genotype (and sometimes also by the environment).
population A group of organisms of a single species living within a certain area.
population genetics The study of the evolution of populations at the level of genes and alleles.
sexual reproduction Two-parent gamete based reproduction, involving meiosis and fer- tilization.
Imagine how Darwin felt, knowing that traits were passed on to offspring and that heritable variations “somehow” appeared. What key discoveries now explain these facts?
As we noted in the last chapter, Theodosius Dobzhansky is famous for his statement, “Nothing in biology makes sense except in the light of evolution.” Do you agree that people cannot understand biology without understanding evolution?
How does evolutionary theory “make sense of” your similarities to your parents and siblings? Your differences from them? Similarities among all humans? Differences among us?
Compare and relate macroevolution to microevolution.
Define microevolution in terms of allele frequencies.
Define genetic equilibrium for a population.
List the five conditions for genetic equilibrium according to the Hardy-Weinberg model.
State and explain the generalized equation for Hardy-Weinberg equilibrium.
Explain how to use the Hardy-Weinberg equation to solve for allele or genotype fre- quencies.
Discuss the reasons why the 5 conditions for Hardy-Weinberg equilibrium are rarely met.
Explain how mutation disrupts genetic equilibrium.
Predict the possible effects of mutation, and analyze the probability of each type of effect.
Contrast mutation in microorganisms to mutation in multicellular organisms.
Define gene flow.
Describe two possible effects of gene flow on the genetics of a population.
Define genetic drift.
Describe three possible effects of genetic drift on populations and/or specific alleles.
Explain and give an example of the bottleneck effect.
Clarify and give an example of the concept of “founder effect.”
Compare and contrast genetic drift and natural selection as causes of evolution.
Discuss natural selection and evolution in terms of phenotypes and allele frequencies.
Explain the distribution of phenotypes for a trait whose genetic basis is polygenic.
Using a normal distribution of phenotypic variation, interpret directional, disruptive, and stabilizing patterns of selection.
Define fitness as it relates to natural selection.
Describe how natural selection can sometimes lead to the persistence of harmful or even lethal allele.
Analyze the logic of kin selection.
How many people in the United States carry the recessive allele for the lethal genetic disease, cystic fibrosis?
Why must we treat AIDS patients with multi-drug “cocktails?”
Why do we consider Northern Elephant seals endangered, even though their population has risen to 100,000 individuals?
Why don’t South African Cheetahs reject skin grafts from unrelated individuals? www.ck12.org 624
What were the probable effects on human evolution of the six years of “volcanic winter” caused by the “megacollosal” eruption of Mt. Toba 70,000 years ago?
Why do humans vary in skin color?
Why doesn’t natural selection eliminate certain lethal genes?
This lesson will introduce you to our current understanding of the causes of evolution and show you how scientists use this understanding to solve problems and answer puzzling ques- tions like those above.
In an earlier chapter, we defined microevolution as evolution within a species or population, and macroevolution as evolution at or above the level of species. The differences between the two are time and scale. Microevolution can refer to changes as small as a shift in allele frequencies for a single gene from one generation to the next. In contrast, macroevolution describes changes in species over geologic time. Many biologists consider evolution to be a single process; they regard macroevolution as the cumulative effects of microevolution. The History of Life chapter discussed patterns and processes of macroevolution; this lesson will focus on genetic changes within populations and species. At this level, we can see how evolution really works – a view that Darwin never had.
A change in allele frequencies within a population from one generation to the next – even if it involves only a single gene - is evolution. Biologists refer to this change in the gene pool as microevolution because it is evolution on the smallest scale. For our rabbit population from the previous lesson, a generation-to-generation increase in the frequency of the albino allele, b, from 0.3 to 0.4 (and the corresponding decrease in the frequency of allele B from
to 0.6) would be microevolution. The changes in Galapagos finch beak size, documented by Peter and Rosemary Grant, and the color changes of peppered moths will undoubtedly show corresponding changes in allele frequencies once we identify the responsible genes and alleles. Resistance to antibiotics in bacteria and the appearance of new strains of influenza viruses and HIV Figure 13.9 also involve changes in allele frequencies. Each of these is an example of microevolution.
What forces cause changes in allele frequencies? What mechanisms determine how mi- croevolution happens? Before we tackle these questions, we will examine a population at equilibrium – a population which is not evolving.
Like Darwin and Wallace, who independently developed similar ideas about evolution and natural selection, mathematician Godfrey Hardy and physician Wilhelm Weinberg indepen- dently developed a model of populations at equilibrium. The Hardy-Weinberg model
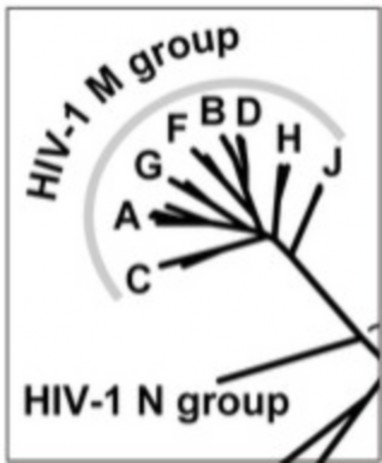
www.ck12.org 626
(sometimes called a law) states that a population will remain at genetic equilibrium - with constant allele and genotype frequencies and no evolution - as long as five conditions are met:
No mutation
No migration
Very large population size
Random mating
No natural selection
We will consider the above in more detail in later sections of the lesson, because deviations from these conditions are the causes of evolutionary change. For now, let’s look more closely at the Hardy-Weinberg’s equilibrium model. We’ll use another hypothetical rabbit popula- tion to make the model concrete: 9 albino rabbits and 91 brown rabbits (49 homozygous and 42 heterozygous). The gene pool contains 140 B alleles and 60 b alleles – gene frequencies of 0.7 and 0.3, respectively. Figure 13.10 summarizes the data for this parent population.
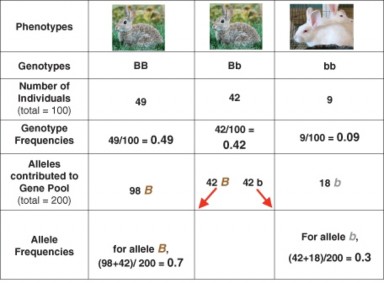
If we assume that alleles sort independently and segregate randomly as sperm and eggs form, and that mating and fertilization are also random, the probability that an offspring will receive a particular allele from the gene pool is identical to the frequency of that allele in the population. A Punnett Square (Figure 13.11) using these frequencies predicts the probability of each genotype (and phenotype) in the next generation:
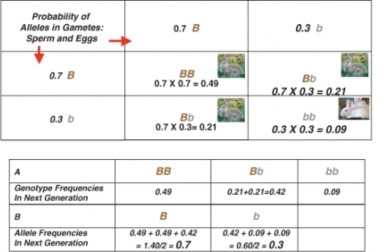
Figure 13.11: A Punnett Square predicts the probability of each genotype and phenotype for the offspring of the population described in Figure 13.10. A summarizes the frequencies of each genotype among offspring, and B calculates allele frequencies for the next generation. Comparing these to the parent generation shows that the gene pool remains constant. The population is stable – at a Hardy-Weinberg equilibrium. (4)
If we calculate the frequency of genotypes among the offspring predicted by the Punnett square, they are identical to the genotype frequencies of the parents. Allele frequency remains constant as well. The population is stable – at a Hardy-Weinberg genetic equilibrium.
A useful equation generalizes the calculations we’ve just completed. Variables include p = the frequency of one allele (we’ll use allele B here) and q = the frequency of the second allele (b, in this example). We will use only two alleles, but similar, valid equations can be written for more alleles.
Recall that the allele frequency equals the probability of any particular gamete receiving that allele. Therefore, when egg and sperm combine, the probability of any genotype (as in the Punnett square above) is the product of the probabilities of the alleles in that genotype. So:
Probability of genotype BB = p X p = p2 and
Probability of genotype Bb= (p X q) + (q X p) = 2 pq and Probability of genotype bb = q X q = q2
We have included all possible genotypes, so the probabilities must add to 1.0. Our equation becomes:
www.ck12.org 628
Table 13.2:
![]()
p2 + 2 pq + q2 = 1
![]()
frequency of geno- type BB
frequency of geno- type Bb
frequency of geno- type bb
![]()
This is the Hardy-Weinberg equation, which describes the relationship between allele fre- quencies and genotype frequencies for a population at equilibrium.
The equation can be used to determine genotype frequencies if allele frequencies are known, or allele frequencies if genotype frequencies are known. Let’s use a common human genetic disease as an example. Cystic fibrosis (CF) is caused by a recessive allele (f ) which makes a non-functional chloride ion channel, leading to excessive mucus in the lungs, inadequate enzyme secretion by the pancreas, and early death (Figure 13.12).
Knowing that 1 of every 3,900 children in the United States is born with CF, we can use the Hardy-Weinberg equation to ask what proportion of the population unknowingly carries the allele for cystic fibrosis. An individual who has CF must have the genotype ff , because the allele is recessive. Using the value for the frequency of the homozygous recessive genotype, we can calculate q, the frequency of the recessive allele,f:
1/3900 = 0.0002564 = frequency of ff genotypes = q2
To find q, the frequency of allele f , we must take the square root of the frequency of ff
genotypes:
q = √0.0002564 = 0.016 = frequency of the f allele
Because p + q = 1 (the sum of the frequency of allele f and the frequency of allele F must equal 1.0),
p = 1 – q = 1.0 – 0.0160 = 0.9840 = frequency of the F allele
According to the Hardy-Weinberg equation, for a population at equilibrium the frequency of carrier genotypes, Ff , is 2pq = 2 X 0.016 X 0.984 = 0.0315.
In other words, if the population is indeed at equilibrium for this gene, just over 3% of the population carries the recessive allele for cystic fibrosis.
Of course, this calculation holds only true if the US population meets the five conditions we listed at the beginning of this section. In nature, populations seldom satisfy all five criteria. Let’s consider how well each condition describes the US population for the cystic fibrosis gene:
Although the equation ideally describes an infinitely large population – never found in nature, of course – the US population is probably large enough that this condition alone does not significantly disrupt equilibrium.
Mutations happen constantly, if at a low rate, so “no mutation” is a second unrealistic condition. However, mutations affecting any one particular gene are rare, so their effect on an otherwise large, stable population is small.
This condition assumes no net additions or losses of either allele to the gene pool through immigration or emigration. For the US population, immigration is probably more significant than emigration. Gene flow, in essence is the flow of alleles into or out of a population, may be the most significant problem for this particular gene, because the frequency of the allele for cystic fibrosis varies greatly according to ancestry. Although 1 in 25 Europeans carry the f allele, the frequency is just 1 in 46 among Hispanics, 1 in 60 among Africans, and 1 in 90 among Asians. Therefore, disproportionate immigration by certain groups changes allele frequencies, destabilizing the Hardy-Weinberg equilibrium.
Here is another assumption which is probably not realistic. Marriage between individuals of similar ancestry and culture is still more common than intermarriage, although both occur.
www.ck12.org 630
If marriages are not random, Hardy-Weinberg equilibrium does not apply.
The final condition is that all genotypes must have an equal chance to survive and reproduce. Victims of cystic fibrosis (genotype ff ) have shorter lifespans, which inevitably reduce re- production compared to individuals without the disease (genotypes FF and Ff ). Although medical care is improving, differential survival and reproduction among genotypes means the gene pool is not at equilibrium.
With respect to the cystic fibrosis gene, the U.S. population fails to meet at least three of the criteria for equilibrium. Therefore, the actual frequencies of alleles and genotypes probably deviate somewhat from those we calculated.
In nature, very few populations meet the Hardy-Weinberg requirements for equilibrium. If we look at this fact from a different angle, we see that any of these conditions can destabilize equilibrium, causing a change in allele frequencies. In other words, these five conditions are five major causes of microevolution. The remaining sections of this lesson will explore each cause in detail.
As discussed in the last lesson, mutation – a random, accidental change in the sequence of nucleotides in DNA – is the original source of genetic variation. Only mutation can create new alleles – new raw material for natural selection. UV or ionizing radiation, chemicals, and viruses constantly generate mutations in a gene pool, destabilizing genetic equilibrium and creating the potential for adaptation to changing environments. However, both rates of mutation and their effects on the fitness of the organism vary.
In multicellular organisms, most mutations occur in body cells and do not affect eggs and sperm; these are lost when the individual dies and usually do not affect evolution. Only mutations in gamete-producing cells can become part of the gene pool. The rate at which mutations enter the gene pool is low, due to DNA “proofreading” and repair enzymes - and the extensive amount of “junk” DNA which does not code for protein. Mutations which do change nucleotide sequences in functional genes may also have no effect, because the Genetic Code is redundant (multiple similar codons for a single amino acid), or very little effect, if the amino acid is not located in a critical part of the protein.
Occasionally, however, a single nucleotide substitution can have a major effect on a protein
as we saw with sickle-cell anemia in the last lesson. Usually, the effect of a mutation on a protein is harmful; rarely is it helpful. In sickle-cell anemia, it is both – in certain environments. Sickled cells carry oxygen much less efficiently, but prevent malaria infections. Overall, the chance that a single mutation will increase the fitness of a multicellular organism is extremely low. If the environment changes, however, the adaptive value of a new allele may change as well. Over time, mutations accumulate, providing the variation needed for
natural selection.
For the small genomes of viruses and bacteria, mutations affect genes directly and generation times are short, so rates of mutation are much higher. For an HIV population in one AIDS patient, rates of viral mutation and replication are so high that in a single day, every site in the HIV genome may have experienced mutation (Figure 13.13). This rapid generation of new alleles challenges our best efforts at drug treatment, and explains the evolution of drug antibiotic resistance. Because of the abundance of random, spontaneous mutations, HIV generates a large amount of raw material for natural selection, and readily evolves resistance to new “environments” created by single drugs (Figure 13.14). Drug “cocktails,” which contain multiple anti-viral chemicals, are our effort to change the “environment” and keep up with mutation in the human-HIV evolutionary race. For microorganisms, mutation is a strong force for evolution.
For all organisms, mutations are the ultimate source of genetic variation. For a population, however, the immigration or emigration of individuals or gametes may also add to or subtract from a gene pool – a process known as gene flow. For example, wind or animals can carry pollen or seeds from one plant population to another. In baboon troops or wolf packs (Figure
www.ck12.org 632
13.15), juvenile males may leave the group to find mates and establish separate populations. Human history includes countless migrations; gene flow continues to mix gene pools and cause microevolutionary change.

Gene flow can bring into a population new alleles which occurred by chance and were success- ful in other populations. In this way, it can accelerate microevolution. However, if exchange between populations is frequent, it reduces differences between populations, in effect increas- ing population size. In this case, gene flow tends to maintain separate populations as one species, reducing speciation, if not microevolution.
Mutation, together with recombination of existing alleles by sexual reproduction (see lesson 13.1) provides the diversity which is the raw material for natural selection and evolution. Gene flow can accelerate the spread of alleles or reduce the differences between populations. Both can contribute significantly to microevolution. However, many biologists consider the major causes of microevolutionary change to be genetic drift and natural selection.
Recall that the third requirement for Hardy-Weinberg equilibrium is a very large population size. This is because chance variations in allele frequencies are minimal in large populations. In small populations, random variations in allele frequencies can significantly influence the “survival” of any allele, regardless of its adaptive value. Random changes in allele frequencies in small populations are known as genetic drift. Many biologists think that genetic drift is a major cause of microevolution.
You see the effects of chance when you flip a coin. If you flipped a penny 4 times, you would not be too surprised if it came up heads 4 times and tails not at all. If you tossed it 100 times, you would be very surprised if the results were 100 heads and no tails. The larger the “population” of coin tosses, the lower the effects of chance, and the closer the results
www.ck12.org 634
should match the expected 50-50 ratio. The same is true for populations. If we imagine a rabbit population with a very small gene pool of just 2 B alleles and 2 b alleles, it is not difficult to understand that occasionally, chance alone would result in no albino offspring (only genotypes BB or Bb) – or even no brown offspring (only genotype bb). However, a gene pool of 100 B alleles and 100 b alleles would be very unlikely to produce a generation of offspring entirely lacking one allele or the other, despite having identical initial allele frequencies of 0.5.
Because chance governs meiosis and fertilization, random variations can influence allele fre- quencies, especially for small populations. Note that these chance variations can increase the frequency of alleles which have no adaptive advantages or disadvantages – or decrease the frequency of alleles which do have adaptive value. Genetic drift can result in extinction of an allele or an entire population – or rapid evolution (Figure 13.16). Two sets of circum- stances can create small populations for which genetic drift can have major consequences: the bottleneck effect and the founder effect.
Natural catastrophes such as earthquakes, floods, fires, or droughts can drastically reduce population size – usually without respect to allele frequencies. As a result of the disaster, some alleles may be lost entirely, and others may be present in frequencies which differ from those of the original population. The smaller population is then subject to genetic drift, which may further reduce diversity within the population. The loss of diversity resulting from a drastic reduction in population size and subsequent genetic drift is the bottleneck effect (Figure 13.17). Much of our concern for endangered species derives from our understanding of the way in which small population size can reduce diversity by increasing genetic drift. We will look at two examples of the bottleneck effect – one caused by humans, and the other probably experienced by our human ancestors.
During the 19th century, overhunting reduced the worldwide population of Northern Elephant Seals (Figure 13.18) to fewer than 100 individuals. Because an alpha bull typically mates with a “harem” of 30-100 females, it is possible that just a single male fathered all the seals which exist today! After legal protection, their numbers have rebounded to 100,000. However, the effects of the past bottleneck - significant loss of genetic variability – remain. Reduced genetic diversity means that today’s seals are more susceptible to disease and weather. Effects of genetic drift on the gene pool may have contributed to the loss of 80% of pups during the El Nino year of 1997-98.
Although the exact cause is unknown, a bottleneck for South African Cheetahs (Figure 13.19) during the last ice age about 10,000 years ago has apparently led to extremely low genetic variability. Genetic variation among cheetahs has been compared to that of highly inbred varieties of laboratory mice; skin grafts between unrelated individuals are not rejected. These animals also suffer from low sperm counts. Like many endangered species, cheetahs
www.ck12.org 636

Figure 13.18: The Northern Elephant Seal population fell to fewer than 100 individuals due to overhunting during the 19th century. Although their numbers have recovered, the bottleneck effect of reduced genetic variation limits their potential to adapt to future environmental changes. (16)
are threatened not only by habitat loss, but also by reduced genetic diversity, which reduces their potential to adapt to changing environments.

We humans may have experienced a population bottleneck between 70,000 and 75,000 years ago, when supervolcano Mount Toba exploded with category 8 (“megacolossal!”) force in Sumatra. According to anthropologist Stanley Ambrose’s theory, global temperature dropped as much as 5 degrees Celsius for several years, possibly leading to an ice age. Ambrose believes that the environmental effects (“six years of relentless volcanic winter”) reduced the total human population to less than 10,000, and that isolated individual popu- lations would have experienced genetic drift and rapid evolution or extinction.
Whereas a drastic reduction in population size causes the bottleneck effect, a form of popu- lation expansion leads to the founder effect. If a small group of individuals (the founders) breaks off from a larger population to colonize a distant area, they will probably carry with them only a limited amount of the genetic diversity of the original population (Figure 13.20). For this reason, the new population they establish may differ significantly in genotype and phenotype. Inevitably, it will also be small and therefore subject to genetic drift.
On newly formed islands, such as the Galapagos, Hawaii, and more recently Surtsey, Iceland, founder populations are often the only source of life on the island. Many founder populations
www.ck12.org 638
probably become extinct, but others evolve rapidly, due to genetic drift. Some may diverge rapidly to occupy many available ecological niches – a process known as adaptive radia- tion. As we have discussed in past chapters, Galapagos finches and Hawaiian honeycreepers probably each evolved from small populations of a single ancestral finch-like species (see the previous chapter on Evolutionary Theory).
Historically and even today, human populations have experienced founder effects. In some cases, migration and colonization are the cause. Quebec was founded by a group of no more than 2,600 people, ancestors of today’s more than 7 million Quebecois, who show remarkable genetic similarity and a number of heritable diseases, well studied by geneticists.
Cultural isolation, as well as colonization, can result in founder effects. Amish populations in the United States have grown from an initial group of about 200 immigrants, dating back to the mid-1700s. Because they have remained culturally and reproductively isolated from non- Amish Americans, they show considerable uniformity. The Amish today are often studied for their genetic uniformity, as well as certain recessive conditions. Geneticists believe that just one or two of the initial 200 Amish carried a recessive allele for Ellis-van Creveld syndrome (short limbs, extra fingers, and heart anomalies), yet through genetic drift, the isolated Amish population now has the highest incidence of this syndrome in the world (Figure 13.21).
While genetic drift, including the bottleneck and founder effects, can cause microevolution (generational change in allele frequencies), its effects are mostly random. The results of genetic drift may include enhanced capabilities, but more often, they are neutral or delete- rious. Natural selection depends not on chance, but on differential survival determined by an individual’s traits. Even though the variations are due to chance, the products of natural selection are usually organisms well-suited to their environment.
Recall that Hardy-Weinberg equilibrium requires that all individuals in a population are equal in their ability to survive and successfully reproduce. As Darwin noted, however, overproduction of offspring and variation among individuals often lead to differential survival and reproduction – in other words, natural selection (Figure 13.22). We discussed natural selection as a part of Darwin’s theory of evolution in the last chapter, but in this section, we will go deeper than Darwin could. We will explore natural selection at the level of populations – in terms of allele, genotype, and phenotype frequencies.
Natural selection acts on an organism’s phenotype (appearance), which is a product of genotype and any environmental influences on gene expression. By selecting for alleles which improve survival and/or reproduction and selecting against harmful alleles, natural selection changes the proportion of alleles from one generation to the next – causing microevolution.
www.ck12.org 640

Figure 13.22: Natural selection involves (1) heritable variation (here, giraffe neck length);
Let’s return once more to our rabbit population. If a predator such as a hawk can see white rabbits (genotype bb) more easily than brown rabbits (BB and Bb), brown rabbits are more likely than white rabbits to survive hawk predation. Because more brown rabbits will survive to reproduce, the next generation will probably contain a higher frequency of B alleles. Note, however, that the recessive b alleles are unlikely to disappear completely, because they can “hide” from the hawks in heterozygous brown rabbits. This is a good reminder that natural selection acts on phenotypes, rather than genotypes. The hawk - or natural selection - is unable to distinguish a BB rabbit from a Bb rabbit. Natural selection - and the hawk - is only able to distinguish a brown rabbit from a white rabbit, demonstrating how natural selection acts on the phenotype rather than the genotype of an organism.
Consider a different example, which emphasizes reproduction rather than survival: If both brown and white rabbits preferred to mate with white rabbits, the next generation’s gene pool would probably show an increase in the frequency of the b allele, because white rabbits would be more likely to reproduce successfully.
Although some traits are determined by a single gene, many are influenced by more than one gene (polygenic). The result of polygenic inheritance is a continuum of phenotypic values which often show a bell curve pattern of variation. Figure 13.24 shows the effect of three genes, each having two alleles, on human skin color; the result is a normal distribution ranging from very dark to very light, with a peak near the middle. You can demonstrate polygenic inheritance (probably with some environmental influence) for height, ear length, or handspan by measuring your classmates and graphing the data in a similar fashion. Some curves will be flat, and others sharp – but most will resemble the normal “bell” shape.
Given this pattern of phenotypic variation, natural selection can take three forms (Figure 13.23). We will use the theoretical human skin color distribution Figure 13.24 to illustrate the three types of selection. Directional selection shifts the frequency curve away from the average by favoring individuals with an extreme form of the variation. The skin of early humans living in sun-rich Africa received high levels of UV radiation, which destroys vitamin B (folate) and leads to severe birth defects such as spina bifida. Selection, then, favored darker-skinned individuals, and the frequency of the darker alleles increased. After several generations, the curve would still be bell-shaped, but it would have shifted to the right, in the direction of the darker alleles. The average individual would have had darker skin as result of this microevolutionary change.
As humans migrated into the northern hemisphere, excessive UV radiation was no longer a problem, but the relative lack of sunlight led to lower levels of vitamin D3, normally syn- thesized in the skin and necessary for calcium absorption and bone growth. Thus, selection in the north favored lighter-skinned individuals – by itself another example of directional selection. However, if we consider the human population as a whole at that time, disrup-
www.ck12.org 642
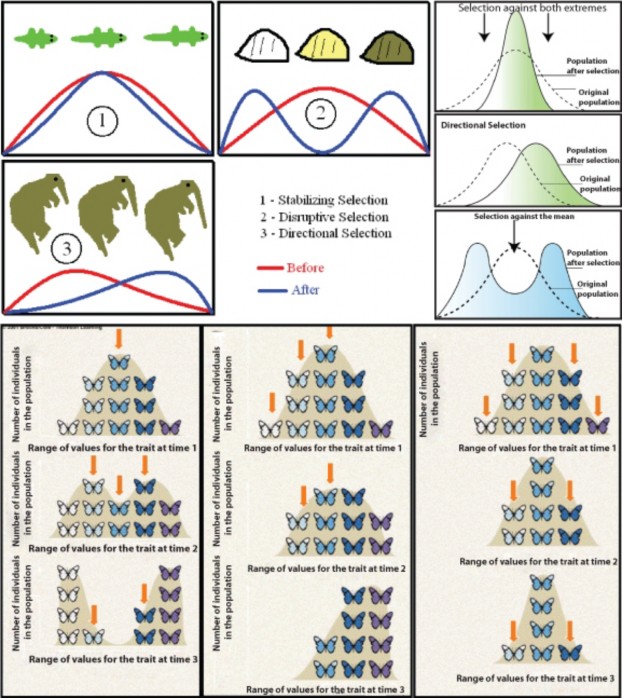
tive selection would describe the microevolution taking place. In northern climates, alleles for light skin would be favored, and in southern climates would select for alleles for dark skin, resulting in two distinct peaks in the distribution of skin color phenotypes and their corresponding genotypes. Keep in mind that the “three gene – dark/light” model is an over- simplification of the genetics underlying skin color, but the adaptive values are real, and the model allows us to illustrate how microevolution works. Note that a map of human skin colors supports this type of selection to some extent (Figure 13.24).
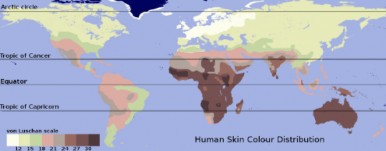
Today, extensive migration, mobility, and intermarriage in the human population may be changing selective pressures on skin color once again. For the sake of argument, let’s make the somewhat unrealistic assumption that mixing becomes complete and that all people will be sufficiently mobile that they experience intermediate levels of sunlight. These conditions would select against both extremely dark skin (too little vitamin D3) and extremely light skin (too little vitamin B-folate). The result would be a taller, narrower distribution – less diversity - about the same mean, a phenomenon known as stabilizing selection. Although our example is perhaps unrealistic, stabilizing selection is probably the most common form of natural selection, preventing form and function from straying away from a “proven” norm.
Stabilizing selection can lead to the preservation of harmful alleles. A famous example, which we considered in earlier lessons, is sickle-cell anemia. The gene for Beta-hemoglobin - half of the oxygen-carrying protein in our blood - has two alleles, which we will call Hgb-A and Hgb-S. Individuals having two copies of the Hgb-S allele suffer from sickle-cell anemia, a potentially lethal disease in which sickled cells clog capillaries and cannot carry oxygen efficiently. In equatorial regions, individuals with two copies of Hgb-A become infected with Plasmodium parasites and often die from malaria. However, individuals with one copy of each allele (the heterozygous genotype) escape both causes of death; although they may experience slight sickling at high altitudes, they do not suffer from full-blown anemia, and
www.ck12.org 644
malaria parasites cannot infect their red blood cells. Stabilizing selection has maintained the frequencies of both alleles, even though each is potentially lethal in the homozygous state.
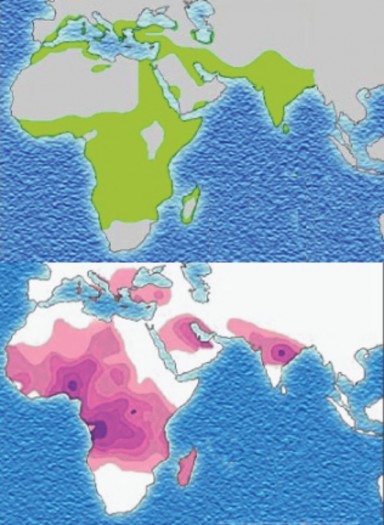
Selection for a particular trait may also select for other traits which do not directly affect fitness – if, for example, genes are linked, or if a single gene influences several different traits.
Another way to look at natural selection is in terms of fitness - the ability of an organism with a certain genotype to reproduce. Fitness can be measured as the proportion of that organism’s genes in all of the next generation’s genes. When differences in individual geno- types affect fitness, the genotypes with higher fitness become more common. This change in genotype frequencies is natural selection.
An intriguing corollary of genotype selection is kin selection. Behaviors which sacrifice reproductive success or even survival can actually increase fitness if they promote the survival and reproduction of close relatives who share a significant proportion of the same genes. Examples include subordinate male turkeys, who help their dominant brothers display to potential mates (Figure 13.26) and honeybee workers, who spend their lives collecting pollen and raising young to ensure that their mother, the queen, reproduces successfully (Figure 13.27).

We have looked carefully at equilibrium populations and at possible disruptions of equilib- rium which cause microevolution – a generational change in a population’s allele frequencies:
Mutation, which together with sexual reproduction is the ultimate source of variation, www.ck12.org 646

and is an important cause of microevolution in microorganisms
Gene flow, which can accelerate microevolution by importing new, already successful alleles
Genetic drift, which can increase the effect of chance variations in small populations
Natural selection, which can be directional, disruptive, or stabilizing
Specialized types of selection, such as mate selection and kin selection
Evolutionary biologists are not yet in agreement regarding the relative importance of each type of selection to the history of life, although most would agree that natural selection is the primary force in microevolution. In the next lesson, we will apply our understanding of microevolutionary processes to that “mystery of mysteries,” as Darwin and Herschel called it: the origin of species.
Macroevolution – change in species over geologic time – is the cumulative effect of microevolution.
Microevolution – evolution within species or populations – can be measured as a generation-to-generation change in allele frequencies.
Non-evolving populations have constant frequencies of alleles and genotypes – genetic equilibrium.
The Hardy-Weinberg model holds for equilibrium populations under 5 conditions:
no mutation
no migration
very large population size
random mating
no natural selection
A generalized form of the Hardy-Weinberg equation for a gene pool of two alleles at equilibrium is:
Table 13.3:
![]()
p2 + 2 pq + q2 = 1
![]()
frequency of geno- type BB
frequency of geno- type Bb
frequency of geno- type bb
![]()
where p is the frequency of one allele and q the frequency of the other allele.
In nature very few populations meet the Hardy-Weinberg requirements for equilib- rium, due to constant mutation, gene flow, small populations, nonrandom mating, and environmental change.
Random, spontaneous mutations constantly generate new alleles in a gene pool, desta- bilizing genetic equilibrium and creating the potential for adaptation to changing en- vironments.
In multicellular organisms, only mutations that affect germ cells become part of the gene pool.
Because of extensive “junk” DNA, repair enzymes, and multicellularity, many muta- tions do not reach the gene pool.
Many mutations are harmful, disrupting protein function.
Because the genetic code is redundant and some amino-acid substitutions may not change protein function, many mutations have no effect on an organism’s fitness.
Even neutral mutations hold potential for future selection if the environment changes.
A few mutations may be advantageous, improving or changing protein function.
In microorganisms mutation rates are much higher due to rapid reproduction and small genomes.
Mutation, together with recombination of existing alleles provides the diversity which www.ck12.org 648
is the raw material for natural selection and evolution.
The movement of genes from one population to another (gene flow) can change allele frequencies.
Gene flow can accelerate the spread of successful alleles or reduce differences between populations.
In small populations random variations in allele frequencies can significantly influence the “survival” of any allele, regardless of its adaptive value; this phenomenon is genetic drift.
Genetic drift can result in extinction of an allele or an entire population.
Alternatively, genetic drift can lead to rapid evolution of a population.
In the bottleneck effect, a catastrophe or disease or overhunting dramatically reduces a population’s size and genetic variation, increasing its susceptibility to the effects of genetic drift.
In the founder effect, a small group leaves a larger population to colonize a new area. Again, genetic drift may lead to loss of genetic diversity, extinction, or rapid evolution.
Genetic drift leads to evolution in populations of small size, but results are mostly due to chance.
Natural selection occurs in populations of any size, and results are more likely to adapt a population to its environment.
Natural selection acts on phenotype variations, so may include environmental effects which are not heritable.
Evolution is a result of natural selection, and is measured as a change in allele frequen- cies.
For a trait whose genetic basis is polygenic, the pattern of phenotypic variation usually forms a bell curve about an average value.
Directional selection results in a shift of allele frequencies toward one extreme.
Disruptive selection favors both extremes over the average phenotypic value.
Stabilizing selection maintains or narrows existing variation in phenotype.
Fitness is the ability of an organism with a certain genotype to reproduce successfully.
Because alleles often affect more than one trait in different ways, or have different effects in different environments, natural selection can sometimes lead to the persistence of harmful or even lethal allele.
Kin selection involves the sacrifice by an individual of his/her reproductive potential in order to help a close relative reproduce successfully.
Define microevolution in terms of allele frequencies.
Describe genetic equilibrium, including the Hardy-Weinberg conditions.
In the United States, about 1 in every 12 blacks of African descent carry one copy of the allele for sickle-cell anemia. Assuming the five conditions of Hardy-Weinberg equilibrium hold (we’ll consider why they probably don’t in question #4), calculate
the probability that a member of the next generation will suffer from sickle-cell anemia (have the homozygous Hemoglobin S genotype).
Discuss the reasons why the 5 conditions for Hardy-Weinberg equilibrium are probably not met by the black population for the Hemoglobin alleles.
Analyze the possible effects of mutation, and the probability and importance of each.
Describe two possible effects of gene flow on the genetics of a population.
Describe three possible effects of genetic drift on populations and/or specific alleles.
Why do we consider Northern Elephant seals endangered, even though their population has risen to 100,000 individuals?
Use the distribution of phenotypes for a trait whose genetic basis is polygenic to inter- pret directional, disruptive, and stabilizing patterns of selection.
Describe how natural selection can sometimes lead to the persistence of harmful or even lethal allele. Include an example.
“The Gene School:”
http://genetics-education-partnership.mbt.washington.edu/class/activities/ HS/sickle-bean.htm
http://www.koshland-science-museum.org/teachers/idactivity-act018.jsp
http://chroma.gs.washington.edu/outreach/genetics/sickle/index.html
http://www.umsl.edu/~biology/hwec/MO-STEP/lessons/naturalsel.html
http://www.phschool.com/science/biology_place/labbench/lab8/intro.html
http://sps.k12.ar.us/massengale/lab_8_sample2_ap_population_gene.htm
http://www.bgsu.edu/departments/chem/faculty/leontis/chem447/PDF_files/Jablonski_ skin_color_2000.pdf
http://www.berkeley.edu/news/media/releases/2005/03/02_turkeys.shtml
http://essp.csumb.edu/eseal/kristi_west/history.html
adaptive radiation Relatively rapid evolution of several species from a single founder population to several to fill a diversity of available ecological niches.
allele frequency The fraction (usually expressed as a decimal) of a population’s gene pool made up of a particular allele.
www.ck12.org 650
bottleneck effect The loss of diversity resulting from a drastic reduction in population size and subsequent genetic drift.
directional selection Selection which favors one side of a phenotypic distribution – one allele or one extreme of a normal distribution.
disruptive selection Selection which favors the two extremes of a phenotypic distribution
– the ends of a bell curve, or the homozygous phenotypes, as opposed to the average, or heterozygous phenotype.
fitness The ability of an organism with a certain genotype to survive and reproduce, often measured as the proportion of that organism’s genes in all of the next generation’s genes.
founder effect The loss of genetic diversity resulting from colonization of a new area by a small group of individuals which have broken off from a larger population.
gene flow The net movement of genes into or out of a population through immigration or emigration.
genetic drift Random changes in allele frequencies in small populations.
genetic equilibrium State of a population in which allele and genotype frequencies remain constant from one generation to the next – a non-evolving population.
Hardy-Weinberg model Describes a population at genetic equilibrium, meeting five con- ditions: no mutation, no migration, very large population size, random mating, and no natural selection.
kin selection Behaviors which sacrifice reproductive success or even survival to promote the survival and reproduction of close relatives who share a significant proportion of the same genes.
macroevolution Evolution at or above the species level.
microevolution Evolution within a species or population, also defined as a generation-to- generation change in allele frequencies for a population.
mutation A change in the nucleotide sequence of DNA or RNA.
natural selection The process by which a certain trait becomes more common within a population, including heritable variation, overproduction of offspring, and differential survival and reproduction.
polygenic trait Traits that are influenced by more than one gene.
stabilizing selection Selection which favors the average or heterozygous phenotype, re- sulting in no change or in a narrowing of the distribution of phenotypes.
This lesson discussed probable past microevolution of alleles for genes for human skin color and hemoglobin. For what other genes (or heritable traits) can you suggest past selective pressures? Do you think certain human genes (or heritable traits) may be at genetic equilibrium? Give some examples, and explain your reasoning.
Some people suggest that we humans have removed ourselves from natural selection. Do you agree?
What are some consequences of understanding that chance variations and natural selection can result in the persistence of lethal alleles, such as the alleles for sickle-cell anemia and cystic fibrosis?
Do you think it is important that people understand the biological basis of skin color? Explain
Recognize that new discoveries since Darwin have added an understanding of speciation to evolutionary theory.
Explain the concept of a species.
Define the biological species concept and analyze its usefulness.
Compare the biological species concept to morphological, genealogical, and ecological concepts.
Analyze the reasons why biologists consider all humans to be members of the same species.
Define speciation.
Describe two conditions that can lead to speciation.
Explain the results of speciation in terms of adaptation, chance, and changes in the environment.
Distinguish allopatric from sympatric speciation. www.ck12.org 652
Describe an experiment which demonstrated allopatric speciation.
Describe two general types of reproductive isolation.
Explain how polyploidy can result in sympatric speciation.
Discuss the use of hybridization to form new crop species.
Analyze the importance of environmental complexity to sympatric speciation for ani- mals.
Compare and contrast the gradualist and punctuated equilibrium models of evolution- ary change.
Describe conditions that could increase the rate of speciation.
Describe circumstances that could lower the rate of speciation.
Darwin avoided the use of the word evolution in his major work about evolution. The word “evolve” appears once, at the very end. He titled his work The Origin of Species – a process or group of processes now called speciation. What exactly is a species? How did the millions of species which exist on earth today arise? Will they (we!) continue to change? How quickly? These are questions Darwin sought to answer – and the questions we will explore in this lesson on evolution. Darwin’s work opened the door to life’s history. This lesson will look at the details and modifications of his ideas which research has made clear in the 150 years since he published The Origin.
A toddler readily recognizes that she/he is surrounded by distinct groups of individuals which we call “kinds” of plants and animals. Biologists refer to kinds of living things as a species - groups of individual organisms which are very similar. If we look closely, however, variations make it quite difficult to decide where to draw the lines between species. For example, are a St. Bernard and a Chihuahua similar enough to group within the same species? Just how similar must two organisms be for biologists to decide they are members of the same species? The answer to this question is not clear, even to biologists who specialize in classification. We will explore several, because how we define a species can help to clarify how species develop.
A widely used definition of species is the biological species concept (Figure 13.28), first proposed by evolutionary biologist Ernst Mayr in 1942. Mayr’s concept begins with the idea that members of a species must be similar enough that they could interbreed. Because all dogs
from a St. Bernard to a Chihuahua – are capable of interbreeding, biologists consider all dogs to be members of the same species. If you are familiar with mules, however, you know
that this definition needs clarification. If horses and donkeys mate, they produce mules, but mules are sterile and cannot continue to interbreed. Therefore, the biological species concept becomes organisms similar enough to interbreed and produce fertile offspring. Horses and donkeys, therefore, are not members of the same species. As you may know, wolves and dogs can interbreed to produce viable hybrids with fertile offspring; surely, wolves and dogs are not members of the same species? The last part of the definition addresses this problem, and the complete definition becomes:
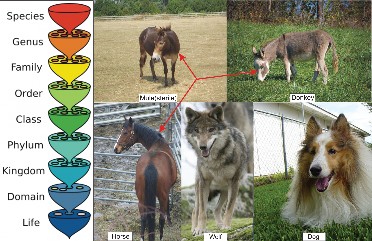
A biological species is a group of organisms similar enough that they could interbreed and produce fertile offspring under natural conditions.
This definition serves the goal of defining members of a species as individuals which are still undergoing evolution – they form a distinct yet potentially common gene pool. You will learn much more about classification in the next chapter, but here it is important to realize that one of the primary goals of classification is to show evolutionary history – patterns of common ancestry. This makes the biological species – a functionally reproducing unit – an important foundation of classification. Closely related species descended from relatively recent common ancestors, and distantly related species descended from more distant common ancestors. The emphasis the biological species concept places on successful reproduction fits this goal of classification quite well.
Another advantage of the biological concept is that it has the potential to explain how www.ck12.org 654
speciation occurred – that is, how two closely related groups of organisms became different species through reproductive isolation. Members of two species may appear very similar, yet fail to interbreed because of reproductive barriers. Barriers to reproduction may either prevent mating, or prevent development of a fertilized egg after mating. Elaborate courtship behaviors, including the blinking pattern of fireflies or the songs of birds, are often required to elicit mating behavior – and limit mating to members of a species (Figure 13.29). Different breeding seasons, such as flowering dates, can also prevent interbreeding. Molecular differences between even closely related species may prevent sperm or pollen from actually fertilizing eggs. Once the eggs are fertilized, chromosomes may be so incompatible that mitosis and meiosis cannot proceed normally; if the zygote cannot develop, offspring do not survive. All of these barriers between species work to ensure successful reproduction within species, keeping specific, useful adaptations “in the family,” so they are a logical way for us, too, to distinguish members of one species from members of another.

Although the biological species concept is extremely helpful in evolutionary thinking it has serious limitations for practical use. For organisms that reproduce asexually – including all bacteria and viruses - the definition is entirely unworkable. Nor can we detect whether or not fossil organisms would have been able to interbreed – whether or not they coexisted. Biologists must rely on structure and (if available) biochemical similarities to classify fossils and most microorganisms.
Alternatives to the biological species concept emphasize the characteristics and processes which unite, rather than divide (reproductive barriers), species. We will look at just a few in order to gain insight into evolutionary thought. A much more practical definition is the morphological species concept, which groups organisms based on structural and biochemical similarities. Recent advances in molecular biology, such as DNA comparison, have strengthened this means of clarifying evolutionary relationships. Biologists probably use this method more than any other to differentiate species in nature, despite its limitations
in confirming the potential for interbreeding.
The ecological species concept focuses on a group’s common ecological niche – the set of environmental conditions and resources used or required by the group. This concept is based on the idea that ecological and evolutionary processes divide resources in such a way that individuals can most efficiently adapt to use those resources as a group. All members of a species, then, have a unique set of adaptations to a particular set of environmental conditions. Note that both the morphological and ecological definitions “work” for asexually reproducing organisms, and many fossils, as well. However, they do not help to explain how two closely related groups became different species, as does the biological definition.
The last concept we will consider has the potential to clarify the path of speciation, or evo- lutionary history – that primary goal of classification. The genealogical or evolutionary species concept defines a species as a group of organisms with a unique genetic history
a group which shares common ancestry without divergence. A species, according to this concept, is a group which forms one tip on the branching tree of life’s history. Modern technology which compares DNA and protein sequences makes this definition workable, but still, the mechanism of speciation is not defined.
Ideally, all of these ways of determining exactly which individuals make up a species would merge; all make valid points about the idea of a species, and all seek the common goal of defining a new, unique unit of life in space and time. Practically, however, each has its own usefulness for different purposes. If we were working in the field, we would undoubtedly use the morphological concept. However, as we explore the idea of speciation mentally and through lab models, we will adopt the biological species concept as our working definition.

www.ck12.org 656
What about humans? Are we all members of the same species? According to the biological species concept, the answer is a resounding yes. Despite our differences in appearance, culture, and location on planet Earth, any human female is capable of producing fertile offspring with any human male. Therefore, all humans are members of the same biological species (Figure 13.30). In contrast, humans and chimpanzees do not interbreed even when they share the same territory, so biologists consider chimpanzees to be a distinct species. Before we leave the “species problem” and Homo sapiens, it is worth noting that all of the various definitions of species confirm the unity of the human species. DNA sequences, cell chemistry, anatomy, ecological resources, and reproductive ability all reveal similarities which unite us as a single species with common ancestry. In fact, the most recent and precise method of comparison – DNA sequences – shows that somewhere between 1 in 100 and 1 in 1,000 base pairs differ, on average, between one human and the next. Based on this analysis, we humans are 99.9% identical. The differences which seem so great to us (skin color, facial features, build, personality) are important for recognition of individuals, and a few may adapt us to particular regions of the Earth, but overall, we are vastly more similar to than different from each other; we are one species.
Although we may not learn exactly how we came to be a separate species, we can gain some insight by looking closely at what is known of the process of speciation. What do we know now – that Darwin did not know - about “the origin of species”?
Speciation occurs at the boundary between microevolution and macroevolution. At what point do the allele frequency changes of microevolution add up to define a new and separate species?
Ernst Mayr, who developed the biological species concept mentioned above, also identified two essential components of speciation. For two populations to evolve into separate species, they must experience:
Isolation
Genetic divergence
Isolation limits gene flow which would tend to maintain uniformity between the two pop- ulations. Physical separation by a barrier limits migration and prevents interbreeding, but differing environmental conditions may also provide a form of isolation. Isolation increases the chance that two populations will take separate microevolutionary pathways. Returning to our white and brown rabbits, even a difference in altitude and thus temperature might separate two parts of a population so that albino individuals will be favored higher on a
mountain where snow frequently accumulates, but brown individuals would be favored down toward the valleys, where snow rarely covers the ground.
Although the two rabbit populations might be isolated by environmental differences, they would not become separate species unless enough genetic differences accumulate to pre- vent successful interbreeding. Genetic divergence is more likely if populations are small, increasing the chance of genetic drift. Even in large populations, however, environmental differences may result in large genetic differences which prevent interbreeding. In our rabbit populations, for example, selection for white rabbits might be accompanied by mate selec- tion based on white coat color. If mate preferences were heritable and sufficiently strong, interbreeding between the brown population and the white population would cease, and the two populations would become two species.
The result of speciation is two groups of individuals, each more closely adapted to local environmental conditions than the larger parent population. Although speciation may not always result in a better fit between a species and its environment (remember that genetic drift increases the influence of chance), over time, natural selection tends to increase fitness. A species’ adaptations to both physical and living environments increase the chance that the species will survive. The millions of species which exist today are each carefully attuned to a particular niche. And, just as variation within a species ensures that some individuals will survive environmental change, a great diversity of species increases the chance that at least some organisms will survive major changes in the environment.
Isolation and genetic divergence are both prerequisites for speciation, but they are so closely related that sometimes it is difficult to distinguish one from the other. Physical isolation may encourage genetic divergence, but many evolutionary biologists believe that reproductive isolation can result from genetic divergence alone. That leads us to two major categories of speciation – one based on geographic isolation, and the other based on reproductive isolation alone. These two categories, and examples of speciation from each, are the topic of the next section.
Biologists today divide isolating mechanisms into two major categories based on whether they happen in different locations (allopatric = “other fatherland”) or a single location (sympatric
= “same fatherland”) (Figure 13.31).
Allopatric speciation involves geographic barriers, which physically isolate populations. Formation of a land bridge such as the Isthmus of Panama, for example, could separate members of a marine population into two groups. Emergence of a mountain range could separate members of a lowland species. According to the fossil record, the rifting of continents
www.ck12.org 658
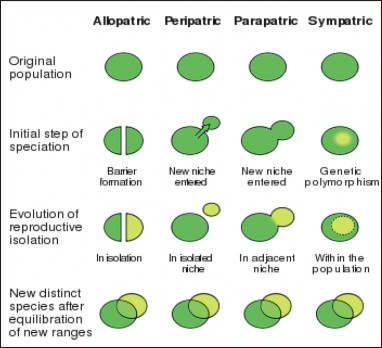
divided populations of terrestrial animals and plants. In these cases, dramatic changes in landforms lead to geographic isolation; however, movements of animals and plants can also result in physical isolation. Single individuals or small groups of individuals may move away from a parent population to colonize a new area, and the new colony could be isolated from its parent population. Such movements are not always intentional; a storm apparently carried a small flock of finches from the coast of South America to the Galapagos Islands. In each case, geographic barriers (the land bridge, the ocean) isolate the two populations so that gene flow stops and genetic divergence can proceed. Natural selection may lead each population to adapt to its own unique environment, or genetic drift may lead to chance differences in gene pools. If genetic divergence results in reproductive incompatibility, the two populations have become two separate species.
Diane Dodd, working with a laboratory population of fruit flies (Figure 13.32), experimen- tally verified the idea that physical isolation can lead to reproductive isolation and speciation. Dodd split the population into two groups, and fed maltose to one group and starch to the other. She observed that each new environment selected for improved efficiency in digesting the available food molecule. After eight or more generations of isolation, the two populations were recombined. After isolation, “starch” flies preferred to mate with other “starch” flies, and “maltose” flies preferred “maltose” mates. Although the preferences were not absolute, they did demonstrate at least initial formation of a reproductive barrier.
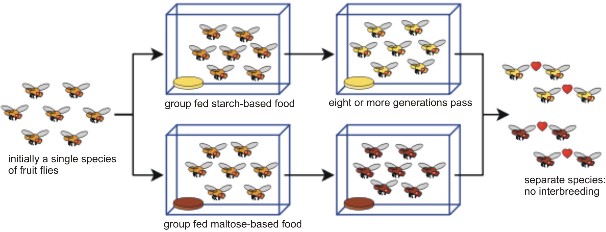
www.ck12.org 660
Sympatric speciation involves the emergence of a new species within the geographic range of the parent population. In the absence of geographic isolation, reproductive barriers must arise in different ways in order for new species to form. Some biologists doubt the relative im- portance of sympatric speciation to evolutionary change, but several examples demonstrate the potential for this mechanism of evolution.
In plants, new species occasionally arise by duplication of chromosomes – a condition known as polyploidy (Figure 13.33). Recall that our human body cells are diploid, and that egg and sperm cells are haploid. If meiosis fails to reduce the number of chromosomes, diploid sex cells result. In plants, which can self-pollinate, diploid pollen may fertilize a diploid egg, resulting in a tetraploid offspring. Although tetraploids may self-pollinate or interbreed with other tetraploids, they cannot successfully reproduce with their parents, because three sets of chromosomes (two from the tetraploid parent and one from the “normal” diploid parent) cannot successfully perform the intricate dance of meiosis. Peanuts, potatoes, and cotton are familiar crops that are tetraploid. Strawberries and sugarcane are octaploid (eight sets of chromosomes in each cell, compared to our two)! Polyploidy is a common form of speciation in plants in nature, as well as in agriculture.
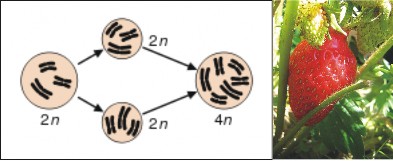
Two species of plants may hybridize – often forming unusually vigorous hybrid offspring. Unfortunately, despite their vigor, these offspring are often infertile due to chromosome incompatibility. However, if polyploidy occurs within the offspring, each of the previously unmatched chromosomes has a partner, offspring fertility is restored, and a new species is formed. Triticale (Figure 13.34), a hexaploid hybrid of wheat and rye, was produced in the
laboratory in this manner. Combining the high yield and quality of wheat with the disease- resistance of rye, triticale is now a successful grain crop in Europe, China, and Australia.

Polyploidy is less common in animals, perhaps because they less frequently self-fertilize. Some salamanders are polyploid, but offspring are usually sterile and “species” reproduce by parthenogenesis (development from unfertilized eggs). Human and other mammalian livers often contain many polyploid cells.
Although polyploidy is rare among animals, other zoological modes of sympatric speciation exist. For example, environmental change within a population’s range may lead to two habitats in which genetic divergence may take place. Our hypothetical rabbit example in the last section is an example of this type of sympatric speciation. A field example involves the hawthorn fly (Figure 13.35), whose larval maggot stage feeds on hawthorn fruit. Apple trees, introduced to the US, often grow near hawthorns, and some hawthorn flies have begun to feed on apples. The two populations, although sympatric, have developed significant genetic differences, and at least in the wild, no longer tend to interbreed. This apparent reproductive isolation involves both temporal divergence (apples and hawthorns and their respective fly populations mature in slightly different seasons) and mating and egg-laying preferences (which link larval fruit to adult behavior). Many biologists consider these flies to be an example of sympatric speciation in progress.
Cichlid fish in East Africa’s Lake Victoria illustrate both allopatric and sympatric speciation (Figure 13.36). Less than a million years old, the Lake harbors nearly 200 closely related cichlid species. Biologists conclude that adaptive radiation by a small group of colonizers
www.ck12.org 662

into available niches explains the diversity of feeding specializations among these closely related species, much like Darwin’s finches in the Galapagos. Because the various habitats in the huge Lake (or islands in the oceans) isolate populations before speciation, adaptive radiation is often considered a type of allopatric speciation. However, nonrandom mating may have led to at least one more recent sympatric speciation. Females of two closely related species appear to select mates based on differing, brightly colored backs. Although the two color variations can still interbreed in the lab, they do not appear to do so in nature.

Speciation and extinction characterize all life on earth; the fossil record clearly documents both. Two startling facts emerge from careful study of the fossil record: First, the average successful species lives for “just” a few million years. Second, over 99.9% of all species that have ever lived have become extinct. The last aspects of speciation that we will consider are the tempo and pattern of species formation.
Over time, geographic changes isolate populations. Small populations experience genetic drift. Mutations alter individual genotypes and gene pools. New habitats form, and small groups colonize them. It is clear that evolution continues to change life. However, there is considerable debate about the rate at which speciation occurs over geologic time. Most biologists agree that single mutations seldom if ever cause new species in single evolutionary
www.ck12.org 664
“leaps.” Mutations in regulatory genes, which have major effects during development, may be an exception, but in general, mutations are more likely to be harmful, and selected against. Except for the special case of polyploidy, discussed above, speciation cannot occur within a single generation. So, what do we know about the rate and pattern of speciation?
Some evolutionary biologists consider the rate of evolution to be slow and constant, with small changes accumulating to form big changes – the idea of gradualism. Others (Niles Eldridge and Stephen Jay Gould), in response to the apparently “sudden” appearance of new forms in the fossil record, suggest that species diverge in bursts of relatively rapid change, and then remain stable for relatively long periods – a model known as punctuated equilibrium. Gould maintains that speciation and evolution occur rapidly in small, peripheral populations, whereas large, central populations remain stabilized for long periods of time. It is the large, central, stable populations which are represented in our fossil record, he argues – not the small, peripheral, evolving ones. The two models are compared in Figure 13.37.
In either case, species require several thousand generations – which may require several thousand years – to form. Relative to the species’ “lifespan” and the modest precision of our ability to pinpoint time within the geologic record, the differences between the two models may be minimal. Major characteristics of species could have evolved gradually over thousands of years, and still we would not be able to detect those changes on the geologic time scale. What we do detect are long periods of relative stability, when stabilizing selection tends to maintain equilibrium. Changes in physiology and internal anatomy could have continued during the long periods of apparent equilibrium without our seeing them; the fossil record cannot reveal these types of changes. The debate is not about the mechanism of evolution
only its tempo.
Proponents of either model admit that the pace of evolution probably changes from one set of conditions to another. Lake Victoria in East Africa, for example, is just a million years old, and yet home to more than 300 species of cichlid fish found nowhere else (return to Figure 13.35). Genetic analysis shows that all of them descended from a single ancestral “founder” species – relatively rapid evolution. Without competition, that species expanded across the huge Lake, and natural selection suited individual populations to exploit the many different food sources and habitats present in the Lake. Cichlid evolution is an example adaptive radiation similar to that of Darwin’s finches and Hawaiian honeycreepers, discussed in the Evolutionary Theory chapter. The fossil record shows similar opportunities for accelerated evolution after mass extinctions. The extinction of the dinosaurs opened an abundance of ecological niches, soon occupied by radiating birds and mammals. Speciation may accelerate not only with a major increase in available niches, but also with the appearance of new adaptation, such as legs or wings, which allows a group to enter a variety of new niches. In contrast, long periods of environmental stability may slow the pace of speciation.
If you look back at the opening questions for this lesson, you can see that both evolution and our investigations into how evolution works are ongoing - works in progress. We have come a long way in understanding “the origin of species” since Darwin, but questions remain. Exactly what is selected - genes, genotypes, phenotypes, or individuals – is still debated;
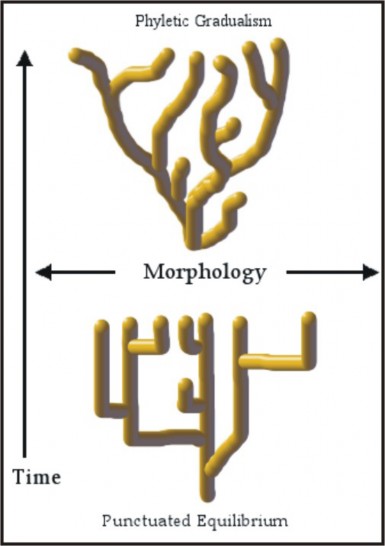
www.ck12.org 666
some, such as Stephen Jay Gould, accept that selection acts at multiple levels. Exactly how fast speciation occurs also remains under discussion; both gradualism and punctuated equilibrium models have merit. Several definitions of a species today serve different purposes; eventually, they should merge into a single clear picture of this basic unit of evolution.
Behind these disagreements, however, lies a powerfully reinforced theory of evolution and an increasingly detailed story of life on Earth. By any definition, humans form a single species, increasingly united by mobility, common genes, and common resources. We and the millions of species with whom we share the Earth today all arose from a single common ancestor billions of years ago – through deep time and myriad instances of allopatric and sympatric speciation. Amazing adaptive logic was most often a result of: directional, stabilizing, and disruptive environmental forces selected for favorable variations and against unfavorable ones. Chance played a major role – through mutation, gene flow, genetic drift, and much environmental change. The pace of speciation probably varied – from gradual change, to rapid change, to long periods of stability. Both speciation and extinction continue today – and will continue into the future, although we cannot predict the direction, because chance determines so much of the direction evolution takes. As a unique species, we are significantly influencing the amount of extinction – and may consequently affect the rate of speciation, as well. That, however, is a topic for a later chapter.
Ever since Darwin’s publication of The Origin of Species, biologists have continued to work out the details of how species originate – the foundation of evolutionary process.
A species is the smallest group of organisms into which biologists classify living things.
A biological species is a group of organisms that can interbreed to produce fertile offspring under natural conditions. This is probably the most widely accepted definition of a species.
A morphological species groups organisms based on extensive structural and biochem- ical similarities. This is probably the most practical definition for use in the field.
A genealogical or evolutionary species includes organisms which share a recent, unique common ancestor. This is the goal of all classification; the only question is how to recognize members.
An ecological species groups organisms together if they share a unique set of adapta- tions to a particular set of environmental conditions.
Ideally, all four definitions would merge to recognize a true species.
All humans are members of the same biological species, because all races and cultures can and do intermarry, and our DNA is 99.9% identical.
For populations to become separate species, they must experience isolation and genetic divergence.
Isolation can be physical or environmental, but it must be accompanied by heritable reproductive isolation, which requires genetic divergence.
The result of speciation is usually two groups of individuals, each more closely adapted to local environmental conditions than the larger parent population.
Because genetic drift and environmental change increase the influence of chance, adap- tation is seldom “perfect” – but over time, natural selection tends to increase fitness.
Allopatric speciation involves geographic barriers, which physically isolate populations.
Barriers to mating and barriers to development of zygotes can both cause reproductive isolation.
Experiments with fruit flies support the possibility that geographic isolation can lead to reproductive isolation.
Sympatric speciation involves the emergence of a new species within the geographic range of the parent population.
In plants, polyploidy is a common sympatric form of “instant speciation.”
Hybridization together with polyploid has formed many vigorous crop species.
In animals, environmental complexity may lead to sympatric speciation.
Cichlid fish in Lake Victoria illustrate both adaptive radiation – a form of allopatric speciation – and more recent sympatric speciation due to heritable changes in mating preference.
The Gradualism model of speciation holds that small changes accumulate to form big changes.
The Punctuated Equilibrium model suggests that rates of change accelerate over short periods in small peripheral populations, and then stabilize for long periods in large, central populations.
Differences between the two may be minimal, because gradual change can occur over thousands of years without our ability to detect it in the fossil record.
Conditions that may accelerate speciation are mass extinctions and the formation of new habitat (e.g. volcanic islands) because both create a sudden availability of many “empty” niches.
Evolution of a major new adaptation, such as legs or wings, may also accelerate speci- ation by suiting a species to multiple new habitats.
Long periods of environmental stability may slow the rate of speciation.
Define the biological species concept and analyze its usefulness.
Compare the biological species concept to morphological, genealogical, and ecological concepts.
Analyze the reasons why biologists consider all humans to be members of the same species.
Describe two conditions that can lead to speciation.
Explain the results of speciation in terms of adaptation, chance, and changes in the environment.
Distinguish allopatric from sympatric speciation. www.ck12.org 668
Describe two general types of reproductive isolation.
Describe the use of hybridization and polyploidy to form new crop species.
Analyze the importance of environmental complexity to sympatric speciation for ani- mals.
Compare and contrast the gradualist and punctuated equilibrium models of evolution- ary change. Give examples which support each.
Dawkins, Richard. The Ancestor’s Tale: A Pilgrimage to the Dawn of Life. 2004. Boston: Houghton-Mifflin, ISBN 0618005838 Punctuated Equilibrium
http://www.pbs.org/wgbh/evolution/library/03/5/l_035_01.html
Phylogenetic Tree
Eastern and Western Meadowlark information, with songs - Cornell Lab of Ornithology
http://www.birds.cornell.edu/AllAboutBirds/BirdGuide/Western_Meadowlark. html
http://www.birds.cornell.edu/AllAboutBirds/BirdGuide/Eastern_Meadowlark.
Adaptive Radiation of Cichlids
http://www.current-biology.com/content/article/fulltext?uid=PIIS0960982207017046
http://www.nature.com/hdy/journal/v99/n4/full/6801047a.html
allopatric speciation The evolution of a new species from a closely related population isolated by geographic barriers.
biological species concept A group of organisms similar enough to interbreed and pro- duce fertile offspring under natural conditions.
ecological niche The set of environmental conditions and resources used or required by a species; the role a species plays in its ecosystem.
ecological species concept A group of organisms which share a unique set of adaptations to a particular set of environmental conditions.
evolutionary species concept See genealogical species concept.
genealogical (evolutionary) species concept A group of organisms which share a re- cent, unique common ancestor – common ancestry without divergence.
gradualism The idea that the tempo of evolution is slow and constant, with small changes accumulating to form big changes.
hybrid Offspring of cross-breeding between two different but closely related species.
morphological species concept A group of organisms which share extensive structural and biochemical similarities.
polyploidy The duplication of chromosome sets, often resulting in “instant speciation.”
punctuated equilibrium The idea that species diverge in bursts of relatively rapid change and then remain stable for relatively long periods.
reproductive barrier A condition which prevents mating or prevents the development of offspring.
reproductive isolation The separation of closely related populations by barriers to pro- ducing viable offspring.
speciation The process which results in new, separate and genetically distinct groups of organisms (species).
species See biological, ecological, genealogical, and morphological species concepts.
sympatric speciation The evolution of new species from closely related populations lo- cated in the same area.
www.ck12.org 670
Which definition of species – biological, morphological, ecological, or genealogical – do you prefer?
To what extent do you think stabilizing, directional, and disruptive selection affect humans today?
What effects might genetic engineering have on speciation?
Do you find the evidence for sympatric speciation (the more disputed of the two forms) convincing?
Are gradualist and punctuated equilibrium models mutually exclusive?
Why don’t disagreements about speciation threaten the theory of evolution by natural selection?
http://commons.wikimedia.org/wiki/Image:MRNA-interaction.png. Public Domain.
National Park Service. http://commons.wikimedia.org/wiki/Image:Wolf_pack_in_Yellowstone_NP.jpg. Public Domain US-NPS.
http://www.flickr.com/photos/zenera/352770831/. Public Domain, CC-BY-SA.
BGA. http://www.flickr.com/photos/benimoto/603735193/. Creative Commons.
Joel Rotunda. http://www.flickr.com/photos/jrotunda85/192554288/. CC-BY-SA.
http://commons.wikimedia.org/wiki/File: Wheat,_rye,_triticale_montage.jpg. GNU-FDL, Public Domain.
Benny Mazur. http://www.flickr.com/photos/benimoto/603735193. CC-BY.
User Toony, GFDL. http://commons.wikimedia.org/wiki/Image:Selection.svg. CC-BY-SA-2.5, 2.0, 1.0.
http://commons.wikimedia.org/wiki/Image:Types-of-mutation.png. CC-BY-2.5, Public Domain NIH.
Ted E. http://commons.wikimedia.org/wiki/Image:Population_bottleneck.svg. GNU-FDL.
http://en.wikipedia.org/wiki/Image:Punctuatedequilibrium.png. PD-User.
Phrood from the Talking Glossary of Genetics.
http://commons.wikimedia.org/wiki/Image:Chromosom.svg. Public Domain.
Renato Bsasutti. http://commons.wikimedia.org/wiki/Image:Map_of_skin_hue_equi3.png. GNU-FDL.
Brenda. http://www.flickr.com/photos/jiggapotpie/328581516/. CC-BY-SA.
http://commons.wikimedia.org/wiki/Image: Kr%C3%B3liki_kalifornijskie_666.jpg. CC-BY-SA-2.5, 2.0, 1.0.
Jan Roletto. http://commons.wikimedia.org/wiki/Image:See_elefanten.jpg. Public Domain.
http://en.wikipedia.org/wiki/Image:Selection_Chart.png. Public Domain.
National Institutes of Health, Department of Health and Human Services. http://commons.wikimedia.org/wiki/Image:HIV_Daughter_Particles.jpg. Public Domain-USGOV-HHS-NIH.
Maureen. http://www.flickr.com/photos/amerune/302221440/. CC-BY.
Ilmari Karonen. http://commons.wikimedia.org/wiki/Image:Speciation_modes.svg. Public Domain.
http://commons.wikimedia.org/wiki/Image:MasaiMaraCheetahs01.jpg. Public Domain.
Shelley Rainey. http://www.flickr.com/photos/85853969@N00/1466951276. CC-BY.
http://commons.wikimedia.org/wiki/Image:HIV-SIV-phylogenetic-tree.png. Public Domain.
Geneviève Baujat and Martine Le Merrer Ellis-Van, Creveld Syndrome, Orphanet Journal of Rare Diseases, 2007. http://commons.wikimedia.org/wiki/Image:Polydactyly_ECS.jpg. CC-BY-2.0.
www.ck12.org 672
BGA. http://www.flickr.com/photos/benimoto/603735193/. Creative Commons.
http://commons.wikimedia.org/wiki/Image:Portrait_of_Muslim_man.jpg http://www.flickr.com/photos/73416633@N00/391989140/ http://www.flickr.com/photos/exfordy/155874181/ http://www.flickr.com/photos/marcalandavis/63268510/. CC-BY-2.0,
CC-BY-SA-2.0, CC-BY, CC-BY, CC-BY, CC-BY.
http://commons.wikimedia.org/wiki/Image: Drosophila_speciation_experiment.svg. Public Domain.
http://commons.wikimedia.org/wiki/File:Mucoviscidose.PNG. The creator of this work allows anyone to use it for any purpose including unrestricted redistribution, commercial use, and modification, GNU Copyright-Free.
http://en.wikipedia.org/wiki/Image:EasternMeadowlark23.jpg. PD USFWS, PD US Gov.
(30) http://www.flickr.com/photos/7193008@N02/449542472/ http://www.flickr.com/photos/wrex/277392736/ http://commons.wikimedia.org/wiki/File:Canis_Lupus_SignatusZOOM.jpg http://www.flickr.com/photos/grendelkhan/106098744/ http://www.flickr.com/photos/35034360660@N01/33791657. CC-BY-SA-2.5.
Kazulanth. http://commons.wikimedia.org/wiki/Image:Founder_effect.png. Public Domain.
http://commons.wikimedia.org/wiki/Image:Hb-animation2.gif http://commons.wikimedia.org/wiki/Image:Redbloodcells.jpg http://commons.wikimedia.org/wiki/Image:Sickle_cell_hemoglobin.png http://commons.wikimedia.org/wiki/Image:Sicklecells.jpg.
CC-BY-SA-2.5,2.0,1.0, Public Domain.
http://commons.wikimedia.org/wiki/Image:Hiv-monotherapy-levels.png. GNU-FDL.
http://commons.wikimedia.org/wiki/Image:Allele-frequency-fr.png. GNU-FDL.
http://en.wikipedia.org/wiki/Image:Sickle_cell_distribution.jpg. CC-BY-SA-2.5, 2.0, 1.0, CC-BY-SA-2.5, 2.0, 1.0.
User Waugsberg, GFDL. http://commons.wikimedia.org/wiki/Image:Bienen_mit_Brut_1.jpg. CC-BY-SA-2.5, 2.0, 1.0.
http://commons.wikimedia.org/wiki/Image:Crataegus-monogyna-frugt.JPG http://commons.wikimedia.org/wiki/Image:Jab%C5%82ko_-_owoc.JPG. (a) Public Domain (b) GNU-FDL (c) CC-BY-SA-2.5.
www.ck12.org 674