Lesson 2.3: Chemical Reactions
Lesson 2.3: Chemical Reactions
Lesson Objectives
Describe the distribution of Earth’s water, and outline the water cycle.
Identify the chemical structure of water, and explain how it relates to water’s unique properties.
Define solution, and describe water’s role as a solvent.
State how water is used to define acids and bases, and identify the pH ranges of acids and bases.
Explain why water is essential for life processes.
Introduction
Water, like carbon, has a special role in biology because of its importance to organisms. Water is essential to all known forms of life. Water, H2O, such a simple molecule, yet it is this simplicity that gives water its unique properties and explains why water is so vital for life.
Water, Water Everywhere
Water is a common chemical substance on Earth. The term water generally refers to its liquid state. Water is a liquid over a wide range of standard temperatures and pressures. However, water can also occur as a solid (ice) or gas (water vapor).
Where Is All the Water?
Of all the water on Earth, about two percent is stored underground in spaces between rocks. A fraction of a percent exists in the air as water vapor, clouds, or precipitation. Another fraction of a percent occurs in the bodies of plants and animals. So where is most of Earth’s water? It’s on the surface of the planet. In fact, water covers about 70 percent of Earth’s surface. Of water on Earth’s surface, 97 percent is salt water, mainly in the ocean. Only 3 percent is freshwater. Most of the freshwater is frozen in glaciers and polar ice caps. The remaining freshwater occurs in rivers, lakes, and other freshwater features.
Although clean freshwater is essential to human life, in many parts of the world it is in short supply. The amount of freshwater is not the issue. There is plenty of freshwater to go around, because water constantly recycles on Earth. However, freshwater is not necessarily located where it is needed, and clean freshwater is not always available.
How Water Recycles
Like other matter on Earth, water is continuously recycled. Individual water molecules are always going through the water cycle (see the Principles of Ecology chapter). In fact, water molecules on Earth have been moving through the water cycle for billions of years. In this cycle, water evaporates from Earth’s surface (or escapes from the surface in other ways), forms clouds, and falls back to the surface as precipitation. This cycle keeps repeating. Several processes change water from one state to another during the water cycle. They include:
Evaporation—Liquid water on Earth’s surface changes into water vapor in the atmo- sphere.
Sublimation—Snow or ice on Earth’s surface changes directly into water vapor in the atmosphere.
Transpiration—Plants give off liquid water, most of which evaporates into the atmo- sphere.
Condensation—Water vapor in the atmosphere changes to liquid water droplets, forming clouds or fog.
Precipitation—Water droplets in clouds are pulled to Earth’s surface by gravity, forming rain, snow, or other type of falling moisture.
Chemical Structure and Properties of Water
You are probably already familiar with many of water’s properties. For example, you no doubt know that water is tasteless, odorless, and transparent. In small quantities, it is also colorless. However, when a large amount of water is observed, as in a lake or the ocean, it is actually light blue in color. These and other properties of water depend on its chemical structure.
The transparency of water is important for organisms that live in water. Because water is transparent, sunlight can pass through it. Sunlight is needed by water plants and other water organisms for photosynthesis (see Biomes, Ecosystems, and Communities chapter).
Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical formula H2O. The arrangement of atoms in a water molecule, shown in Figure 2.21, explains many of water’s chemical properties. In each water molecule, the nucleus of the oxygen atom (with 8 positively charged protons) attracts electrons much more strongly than do the hydrogen nuclei (with only one positively charged proton). This results in a negative electrical charge near the oxygen atom (due to the ”pull” of the negatively charged electrons toward the oxygen nucleus) and a positive electrical charge near the hydrogen atoms. A difference in electrical charge between different parts of a molecule is called polarity. A polar molecule is a molecule in which part of the molecule is positively charged and part of the molecule is negatively charged.
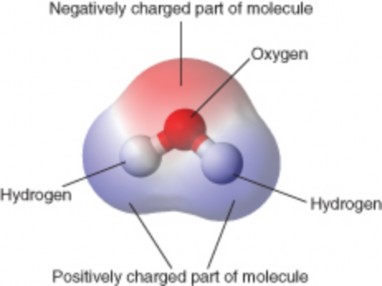
Opposite electrical charges attract one another other. Therefore, the positive part of one water molecule is attracted to the negative parts of other water molecules. Because of this attraction, bonds form between hydrogen and oxygen atoms of adjacent water molecules, as demonstrated in Figure 2.22. This type of bond always involves a hydrogen atom, so it is called a hydrogen bond. Hydrogen bonds are bonds between molecules, and they are not as strong as bonds within molecules. Nonetheless, they help hold water molecules together.
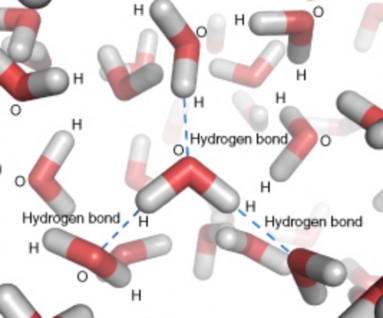
Hydrogen bonds can also form within a single large organic molecule (see the Organic Com- pounds lesson). For example, hydrogen bonds that form between different parts of a protein molecule bend the molecule into a distinctive shape, which is important for the protein’s functions. Hydrogen bonds also hold together the two nucleotide chains of a DNA molecule.
Sticky, Wet Water
Water has some unusual properties due to its hydrogen bonds. One property is the tendency for water molecules to stick together. For example, if you drop a tiny amount of water onto a very smooth surface, the water molecules will stick together and form a droplet, rather than spread out over the surface. The same thing happens when water slowly drips from a leaky faucet. The water doesn’t fall from the faucet as individual water molecules but as droplets of water. The tendency of water to stick together in droplets is also illustrated by the dew drops in Figure 2.23.
Hydrogen bonds also explain why water’s boiling point (100° C) is higher than the boiling points of similar substances without hydrogen bonds. Because of water’s relatively high boiling point, most water exists in a liquid state on Earth. Liquid water is needed by all living organisms. Therefore, the availability of liquid water enables life to survive over much of the planet.

Density of Ice and Water
The melting point of water is 0° C. Below this temperature, water is a solid (ice). Unlike most chemical substances, water in a solid state has a lower density than water in a liquid state. This is because water expands when it freezes. Again, hydrogen bonding is the reason. Hydrogen bonds cause water molecules to line up less efficiently in ice than in liquid water. As a result, water molecules are spaced farther apart in ice, giving ice a lower density than liquid water. A substance with lower density floats on a substance with higher density. This explains why ice floats on liquid water, whereas many other solids sink to the bottom of liquid water.
In a large body of water, such as a lake or the ocean, the water with the greatest density always sinks to the bottom. Water is most dense at about 4° C. As a result, the water at the bottom of a lake or the ocean usually has temperature of about 4° C. In climates with cold winters, this layer of 4° C water insulates the bottom of a lake from freezing temperatures. Lake organisms such as fish can survive the winter by staying in this cold, but unfrozen, water at the bottom of the lake.
Solutions
Water is one of the most common ingredients in solutions. A solution is a homogeneous mixture composed of two or more substances. In a solution, one substance is dissolved in another substance, forming a mixture that has the same proportion of substances throughout. The dissolved substance in a solution is called the solute. The substance in which is it dissolved is called the solvent. An example of a solution in which water is the solvent is salt water. In this solution, a solid—sodium chloride—is the solute. In addition to a solid dissolved in a liquid, solutions can also form with solutes and solvents in other states of matter. Examples are given in Table 1.
Table 2.3:
![]()
![]()
Solvent Gas Liquid Solid Gas Oxygen and other
gases in nitrogen (air)
Liquid Carbon dioxide in water (carbonated water)
Ethanol (an alcohol) in water
Sodium chloride in water (salt water)
Solid Hydrogen in metals Mercury in silver and other metals (dental fillings)
Iron in carbon (steel)
![]()
(Source: http://en.wikipedia.org/wiki/Solute, License: Creative Commons)
The ability of a solute to dissolve in a particular solvent is called solubility. Many chemical substances are soluble in water. In fact, so many substances are soluble in water that water is called the universal solvent. Water is a strongly polar solvent, and polar solvents are better at dissolving polar solutes. Many organic compounds and other important biochemicals are polar, so they dissolve well in water. On the other hand, strongly polar solvents like water cannot dissolve strongly nonpolar solutes like oil. Did you ever try to mix oil and water? Even after being well shaken, the two substances quickly separate into distinct layers.
Acids and Bases
Water is the solvent in solutions called acids and bases. To understand acids and bases, it is important to know more about pure water, in which nothing is dissolved. In pure water (such as distilled water), a tiny fraction of water molecules naturally breaks down, or dissociates, to form ions. An ion is an electrically charged atom or molecule. The dissociation of pure water into ions is represented by the chemical equation:
2 H2O → H3O+ + OH-.
The products of this reaction are a hydronium ion (H3O+) and a hydroxide ion (OH-). The hydroxide ion is negatively charged. It forms when a water molecule donates, or gives up, a positively charged hydrogen ion. The hydronium ion, modeled in Figure 2.24, is positively charged. It forms when a water molecule accepts a positively charged hydrogen ion (H+).
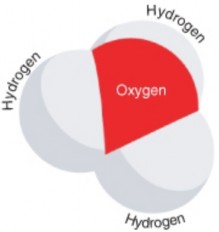
Figure 2.24: A hydronium ion has the chemical formula H3O+. The plus sign (+) indicates that the ion is positively charged. How does this molecule differ from the water molecule in Figure 2.21? (8)
Acidity and pH
Acidity refers to the hydronium ion concentration of a solution. It is measured by pH. In pure water, the hydronium ion concentration is very low. Only about one in ten million water molecules naturally dissociates to form a hydronium ion in pure water. This gives water a pH of 7. The hydronium ions in pure water are also balanced by hydroxide ions, so pure water is neutral (neither an acid nor a base).
Because pure water is neutral, any other solution with the same hydronium ion concentration and pH is also considered to be neutral. If a solution has a higher concentration of hydronium ions and lower pH than pure water, it is called an acid. If a solution has a lower concentration of hydronium ions and higher pH than pure water, it is called a base. Several acids and bases and their pH values are identified on the pH scale, which ranges from 0 to 14, in Figure 2.25.
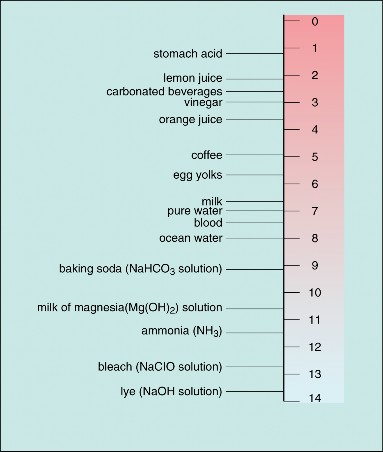
Figure 2.25: Acidity and the pH Scale Water has a pH of 7, so this is the point of neutrality on the pH scale. Acids have a pH less than 7, and bases have a pH greater than 7. The pH scale is a negative logarithmic scale. Because the scale is negative, as the ion concentration increases, the pH value decreases. In other words, the more acidic the solution, the lower the pH value. Because the scale is logarithmic, each one-point change in pH reflects a ten-fold change in the hydronium ion concentration and acidity. For example, a solution with a pH of 6 is ten times as acidic as pure water with a pH of 7.
Acids
An acid can be defined as a hydrogen ion donor. The hydrogen ions bond with water molecules, leading to a higher concentration of hydronium ions than in pure water. For example, when hydrochloric acid (HCl) dissolves in pure water, it donates hydrogen ions (H+) to water molecules, forming hydronium ions (H3O+) and chloride ions (Cl-). This is represented by the chemical equation:
HCl + H2O → Cl- + H3O+.
Strong acids can be harmful to organisms and damaging to materials. Acids have a sour taste and may sting or burn the skin. Testing solutions with litmus paper is an easy way to identify acids. Acids turn blue litmus paper red.
Bases
A base can be defined as a hydrogen ion acceptor. It accepts hydrogen ions from hydronium ions, leading to a lower concentration of hydronium ions than in pure water. For example, when the base ammonia (NH3) dissolves in pure water, it accepts hydrogen ions (H+) from hydronium ions (H3O+) to form ammonium ions (NH4+) and hydroxide ions (OH-). This is represented by the chemical equation:
NH3 + H2O → NH4+ + OH-.
Like strong acids, strong bases can be harmful to organisms and damaging to materials. Bases have a bitter taste and feel slimy to the touch. They can also burn the skin. Bases, like acids, can be identified with litmus paper. Bases turn red litmus paper blue.
Neutralization
What do you think would happen if you mixed an acid and a base? If you think the acid and base would “cancel each other out,” you are right. When an acid and base react, they form a neutral solution of water and a salt (a molecule composed of a positive and negative ion). This type of reaction is called a neutralization reaction. For example, when the base sodium hydroxide (NaOH) and hydrochloric acid (HCl) react, they form a neutral solution of water and the salt sodium chloride (NaCl). This reaction is represented by the chemical equation:
NaOH + HCl → NaCl + H2O.
In this reaction, hydroxide ions (OH-) from the base combine with hydrogen ions (H+) from the acid to form water. The other ions in the solution (Na+) and (Cl-) combine to form sodium chloride.
Acids and Bases in Organisms
Enzymes are needed to speed up biochemical reactions. Most enzymes require a specific range of pH in order to do their job. For example, the enzyme pepsin, which helps break down proteins in the human stomach, requires a very acidic environment in order to function. Strong acid is secreted into the stomach, allowing pepsin to work. Once the contents of the stomach enter the small intestine, where most digestion occurs, the acid must be neutralized. This is because enzymes that work in the small intestine need a basic environment. An organ near the small intestine, called the pancreas, secretes bicarbonate ions (HCO3-) into the small intestine to neutralize the stomach acid.
Bicarbonate ions play an important role in neutralizing acids throughout the body. Bicar- bonate ions are especially important for protecting tissues of the central nervous system from changes in pH. The central nervous system includes the brain, which is the body’s control center. If pH deviates too far from normal, the central nervous system cannot function properly. This can have a drastic effect on the rest of the body.
Water and Life
Humans are composed of about 70 percent water (not counting water in body fat). This water is crucial for normal functioning of the body. Water’s ability to dissolve most biologically significant compounds—from inorganic salts to large organic molecules—makes it a vital solvent inside organisms and cells.
Water is an essential part of most metabolic processes within organisms. Metabolism is the sum total of all body reactions, including those that build up molecules (anabolic reactions) and those that break down molecules (catabolic reactions). In anabolic reactions, water is generally removed from small molecules in order to make larger molecules. In catabolic reactions, water is used to break bonds in larger molecules in order to make smaller molecules.
Water is central to two related, fundamental metabolic reactions in organisms: photosynthe- sis (Photosynthesis chapter) and respiration (Cellular Respiration chapter). All organisms depend directly or indirectly on these two reactions.
In photosynthesis, cells use the energy in sunlight to change water and carbon dioxide into glucose and oxygen. This is an anabolic reaction, represented by the chemical equation:
6 CO2 + 6 H2O + energy → C6H12O6, + 6 O2.
In cellular respiration, cells break down glucose in the presence of oxygen and release energy, water, and carbon dioxide. This is a catabolic reaction, represented by the chemical equation:
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + energy
Two other types of reactions that occur in organisms and involve water are dehydration and hydration reactions. A dehydration reaction occurs when molecules combine to form a single, larger molecule and also a molecule of water. (If some other small molecule is formed instead of water, the reaction is called by the more general term, condensation reaction.) It is a type of catabolic reaction. An example of a dehydration reaction is the formation of peptide bonds between amino acids in a polypeptide chain. When two amino acids bond together, a molecule of water is lost. This is shown in Figure 2.26.
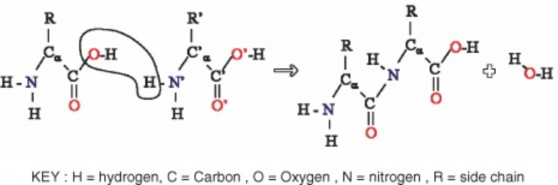
A hydration reaction is the opposite of a dehydration reaction. A hydration reaction adds water to an organic molecule and breaks the large molecule into smaller molecules. Hydration reactions occur in an acidic water solution. An example of hydration reaction is the breaking of peptide bonds in polypeptides. A hydroxide ion (OH-) and a hydrogen ion (H+) (both from a water molecule) bond to the carbon atoms that formed the peptide bond. This breaks the peptide bond and results in two amino acids.
Water is essential for all of these important chemical reactions in organisms. As a result, virtually all life processes depend on water. Clearly, without water, life as we know it could not exist.
Lesson Summary
Most of Earth’s water is salt water located on the planet’s surface. Water is constantly recycled through the water cycle.
Water molecules are polar, so they form hydrogen bonds. This gives water unique properties, such as a relatively high boiling point.
A solution is a homogeneous mixture in which a solute dissolves in a solvent. Water is a very common solvent, especially in organisms.
The ion concentration of neutral, pure water gives water a pH of 7 and sets the standard for defining acids and bases. Acids have a pH lower than 7, and bases have a pH higher than 7.
Water is essential for most life processes, including photosynthesis, cellular respiration, and other important chemical reactions that occur in organisms.
Review Questions
Further Reading / Supplemental Links
Where is most of Earth’s water?
What is polarity, and why is water polar?
Define solution, and give an example of a solution.
What is the pH of a neutral solution? Why?
Draw a circle diagram to represent the water cycle. Identify the states of water and the processes in which water changes state throughout the cycle.
What type of reaction is represented by the chemical equation below? Defend your answer. KOH + HCl → KCl + H2O
Explain how hydrogen bonds cause molecules of liquid water to stick together.
Summarize how metabolism in organisms depends on water.
Philip Ball, Life’s Matrix: A Biography of Water. University of California Press, 2001.
Robert A. Copeland, Enzymes: A Practical Introduction to Structure, Mechanisms, and Data Analysis. Wiley, 2000.
Peter Swanson, Water: The Drop of Life. Cowles Creative Publishing, 2001.
www.infoplease.com/cig/biology/organic-chemistry.html
http://en.wikibooks.org/wiki/Organic_Chemistry/Introduction_to_reactions/ Alkyne_hydration
Vocabulary
acid Solution with a higher hydronium ion concentration than pure water and a pH lower than 7.
acidity Hydronium ion concentration of a solution.
base Solution with a lower hydronium ion concentration than pure water and a pH higher than 7.
condensation Process in which water vapor changes to water droplets, forming clouds or fog.
evaporation Process in which liquid water changes into water vapor.
hydrogen bond Bond that forms between a hydrogen atom in one molecule and a different atom in another molecule.
ion Electrically charged atom or molecule.
metabolism Sum total of all body reactions, including those that build up molecules (anabolic reactions) and those that break down molecules (catabolic reactions).
neutralization Chemical reaction in which an acid and a base react to form a neutral solution of water and a salt.
pH Measure of the acidity, or hydronium ion concentration, of a solution. polarity Difference in electrical charge between different parts of a molecule. precipitation Rain, snow, sleet, or other type of moisture that falls from clouds. solubility Ability of a solute to dissolve in a particular solvent.
solute Substance in a solution that is dissolved by the other substance (the solvent). solution Homogeneous mixture in which one substance is dissolved in another. solvent Substance in a solution that dissolves the other substance (the solute). sublimation Process in which snow or ice changes directly into water vapor.
transpiration Process in which plants give off water, most of which evaporates.
Points to Consider
Most life processes take place within cells. You probably know that cells are the microscopic building blocks of organisms.
What do you think you would see if you could look inside a cell?
What structures might you see?
What processes might you observe?
Image Sources
CK-12 Foundation. http://commons.wikimedia.org/wiki/File:PH_scale.png. CC-BY-SA.
http://en.wikipedia.org/wiki/Image:DNA_chemical_structure.svg. GNU FDL.
CK-12 Foundation. The Periodic Table.. Public Domain.
CK-12 Foundation. States of Matter. CC-BY-SA.
http://commons.wikimedia.org/wiki/Image:Glucose.png. Creative Commons.
http://en.wikipedia.org/wiki/Image:Bouncing_ball_strobe_edit.jpg. Creative Commons.
http://commons.wikimedia.org/wiki/File:Hydronium.png. Public Domain.
http://en.wikipedia.org/wiki/Image:Double_Helix.png. Public Domain.
http://en.wikipedia.org/wiki/Image:Protein-structure.png. Public Domain.
http://en.wikipedia.org/wiki/Image:Liquid_water_hydrogen_bond.png. GNU-FDL.
http://commons.wikimedia.org/wiki/Image: Fat_triglyceride_shorthand_formula.PNG. Pubic Domain.
http://en.wikipedia.org/wiki/Image:Activation2_updated.svg. GNU-FDL.
http://en.wikipedia.org/wiki/Image:Saccharose.svg. Creative Commons.
http://commons.wikimedia.org/wiki/Image:Stylised_Lithium_Atom.png. Creative Commons.
http://en.wikipedia.org/wiki/Image:Water_molecule.svg. Creative Commons.
http://en.wikipedia.org/wiki/Image:AminoAcidball.svg. Creative Commons.
http://en.wikipedia.org/wiki/Image:Water_drops_on_spider_web.jpg. Public Domain.
http://en.wikipedia.org/wiki/Image:2-amino-acidsb.png. Public Domain.
http://en.wikipedia.org/wiki/Image:
Water-elpot-transparent-3D-balls.png. Public Domain.
http://en.wikipedia.org/wiki/Image:Protein-primary-structure.png. Public Domain.
http://en.wikipedia.org/wiki/Image:Rasyslami.jpg. Creative Commons.
http://commons.wikimedia.org/wiki/Image:DNA_ORF.gif. Public Domain.
http://commons.wikimedia.org/wiki/File:Genetic_code.svg. CC-BY-SA.
- Log in or register to post comments
- Email this page
