Lesson 2.1: Matter
Lesson 2.1: Matter
Lesson Objectives
Describe elements and compounds, and explain how mixtures differ from compounds.
Define energy, and describe how energy can be changed from one form to another.
Identify three states of matter, and explain how they differ.
Introduction
Living things are made of matter. In fact, matter is the “stuff” of which all things are made. Anything that occupies space and has mass is known as matter. Matter, in turn, consists of chemical substances.
Chemical Substances
A chemical substance is a material that has a definite chemical composition. It is also ho- mogeneous, so the same chemical composition is found uniformly throughout the substance. A chemical substance may be an element or a chemical compound.
Elements
An element is a pure substance that cannot be broken down into different types of sub- stances. Examples of elements include carbon, oxygen, hydrogen, and iron. Each element is made up of just one type of atom. An atom is the smallest particle of an element that still characterizes the element. As shown in Figure 2.1, at the center of an atom is a nucleus. The nucleus contains positively charged particles called protons and electrically neutral par- ticles called neutrons. Surrounding the nucleus is a much larger electron cloud consisting of negatively charged electrons. An atom is electrically neutral if it has the same number of protons as electrons. Each element has atoms with a characteristic number of protons. For example, all carbon atoms have six protons, and all oxygen atoms have eight protons.
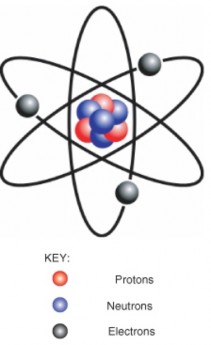
Figure 2.1: Model of an Atom. The protons and neutrons of this atom make up its nucleus. Electrons surround the nucleus. KEY: Red = protons, Blue = neutrons, Black = electrons. (16)
There are almost 120 known elements (Figure 2.2). The majority of known elements are classified as metals. Metals are elements that are lustrous, or shiny. They are also good conductors of electricity and heat. Examples of metals include iron, gold, and copper. Fewer than 20 elements are classified as nonmetals. Nonmetals lack the properties of metals. Examples of nonmetals include oxygen, hydrogen, and sulfur. Certain other elements have properties of both metals and nonmetals. They are known as metalloids. Examples of metalloids include silicon and boron.
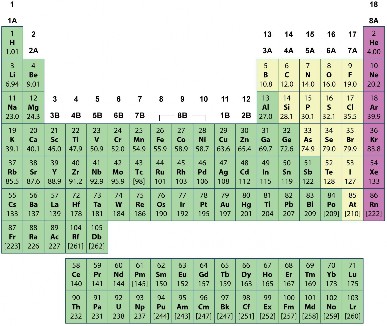
Figure 2.2: The Periodic Table. (3)
Chemical Compounds
A chemical compound is a new substance that forms when atoms of two or more elements react with one another. A chemical reaction is a process that changes some chemical sub- stances into other chemical substances. A compound that results from a chemical reaction always has a unique and fixed chemical composition. The substances in the compound can be separated from one another only by another chemical reaction. This is covered further in the Chemical Reactions lesson.
The atoms of a compound are held together by chemical bonds. Chemical bonds form when atoms share electrons. There are different types of chemical bonds, and they vary in how strongly they hold together the atoms of a compound. Two of the strongest types of bonds are covalent and ionic bonds. Covalent bonds form between atoms that have little if any difference in electronegativity. Electronegativity is the power of an atom to attract electrons toward itself. Ionic bonds, in contrast, form between atoms that are significantly different in electronegativity.
An example of a chemical compound is water. A water molecule forms when oxygen (O) and hydrogen (H) atoms react and are held together by covalent bonds. Like other compounds, water always has the same chemical composition: a 2:1 ratio of hydrogen atoms to oxygen atoms. This is expressed in the chemical formula H2O. A model of a water molecule is shown in Figure 2.3.
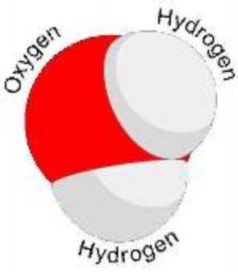
Figure 2.3: Model of a water molecule, showing the arrangement of hydrogen and oxygen atoms (17)
Compounds that contain mainly the elements carbon and hydrogen are called organic compounds. This is because they are found mainly in living organisms. Most organic compounds are held together by covalent bonds. An example of an organic compound is glucose (C6H12O6), which is shown in Figure 2.4. Glucose is a simple sugar that living cells use for energy. All other compounds are called inorganic compounds. Water is an example of an inorganic compound. You will read more about organic compounds in Lesson 2.2.
Mixtures vs. Compounds
Like a chemical compound, a mixture consists of more than one chemical substance. Unlike a compound, a mixture does not have a fixed chemical composition. The substances in a mixture can be combined in any proportions. A mixture also does not involve a chemical reaction. Therefore, the substances in a mixture are not changed into unique new substances, and they can be separated from each other without a chemical reaction.
The following examples illustrate these differences between mixtures and compounds. Both examples involve the same two elements: the metal iron (Fe) and the nonmetal sulfur (S).
When iron filings and sulfur powder are mixed together in any ratio, they form a mixture. No chemical reaction occurs, and both elements retain their individual prop- erties. A magnet can be used to mechanically separate the two elements by attracting the iron filings out of the mixture and leaving the sulfur behind.
When iron and sulfur are mixed together in a certain ratio and heated, a chemical reaction occurs. This results in the formation of a unique new compound, called iron
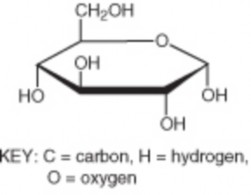
Figure 2.4: Glucose Molecule. This model represents a molecule of glucose, an organic compound composed of carbon, hydrogen, and oxygen. The chemical formula for glucose is C6H12O6. This means that each molecule of glucose contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. NOTE: Each unlabeled point where lines intersect represents another carbon atom. Some of these carbons and the oxygen atom are bonded to another hydrogen atom, not shown here. (6)
sulfide (FeS). A magnet cannot be used to mechanically separate the iron from the iron sulfide because metallic iron does not exist in the compound. Instead, another chemical reaction is required to separate the iron and sulfur.
Matter and Energy
Energy is a property of matter that is defined as the ability to do work. The concept of energy is useful for explaining and predicting most natural phenomena, and it is foundational for an understanding of biology. All living organisms need energy to grow and reproduce. However, energy can never be created or destroyed. It is always conserved. This is called the law of conservation of energy. Therefore, organisms cannot create the energy they need. Instead, they must obtain energy from the environment. Organisms also cannot destroy or use up the energy they obtain. They can only change it from one form to another.
Forms of Energy
Energy can take several different forms. Common forms of energy include light, chemical, and heat energy. Other common forms are kinetic and potential energy.
How Organisms Change Energy
In organisms, energy is always changing from one form to another. For example, plants ob- tain light energy from sunlight and change it to chemical energy in food molecules. Chemical energy is energy stored in bonds between atoms within food molecules. When other organ- isms eat and digest the food, they break the chemical bonds and release the chemical energy. Organisms do not use energy very efficiently. About 90 percent of the energy they obtain from food is converted to heat energy that is given off to the environment.
Kinetic and Potential Energy
Energy also constantly changes back and forth between kinetic and potential energy. Kinetic energy is the energy of movement. For example, a ball falling through the air has kinetic energy because it is moving (Figure 2.5). Potential energy is the energy stored in an object due to its position. A bouncing ball at the top of a bounce, just before it starts to fall, has potential energy. For that instant, the ball is not moving, but it has the potential to move because gravity is pulling on it. Once the ball starts to fall, the potential energy changes to kinetic energy. When the ball hits the ground, it gains potential energy from the impact. The potential energy changes to kinetic energy when the ball bounces back up into the air. As the ball gains height, it regains potential energy because of gravity.
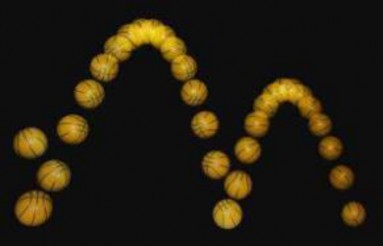
Figure 2.5: Energy in a bouncing ball is transformed from potential energy to kinetic energy and then back to potential energy. This cycle of energy changes keeps repeating as long as the ball continues to bounce. The ball rises less on each successive bounce because some energy is used to resist air molecules. (7)
Like the ball, every time you move you have kinetic energy — whether you jump or run or just blink your eyes. Can you think of situations in which you have potential energy? Obvious examples might include when you are standing on a diving board or at the top of a ski slope or bungee jump. What gives you potential energy in all of these situations? The answer is gravity.
States of Matter
The amount of energy in molecules of matter determines the state of matter. Matter can exist in one of several different states, including a gas, liquid, or solid state. These different states of matter have different properties, which are illustrated in Figure 2.6.
A gas is a state of matter in which atoms or molecules have enough energy to move freely. The molecules come into contact with one another only when they randomly collide. Forces between atoms or molecules are not strong enough to hold them to- gether.
A liquid is a state of matter in which atoms or molecules are constantly in contact but have enough energy to keep changing positions relative to one another. Forces between atoms or molecules are strong enough to keep the molecules together but not strong enough to prevent them from moving.
A solid is a state of matter in which atoms or molecules do not have enough energy to move. They are constantly in contact and in fixed positions relative to one another.
Forces between atoms or molecules are strong enough to keep the molecules together and to prevent them from moving.
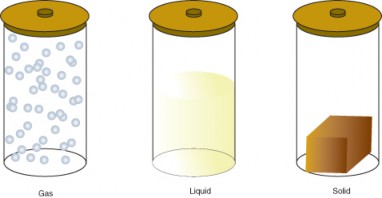
Figure 2.6: States of Matter. (4)
All three containers contain a substance with the same mass, but the substances are in different states. In the left-hand container, the substance is a gas, which has spread to fill its container. It takes both the shape and volume of the container. In the middle container, the substance is a liquid, which has spread to take the shape of its container but not the volume. In the right-hand container, the substance is a solid, which takes neither the shape nor the volume of its container.
What Determines a Substance’s State?
Which state a substance is in depends partly on temperature and air pressure. For example, at the air pressure found at sea level, water exists as a liquid at temperatures between 0° C and 100° C. Above 100° C, water exists as a gas (water vapor). Below 0° C, water exists as a solid (ice). Different substances have a different range of temperatures at which they exist in each state. For example, oxygen is gas above -183° C, but iron is a gas only above 2861° C. These differences explain why some substances are always solids at normal Earth temperatures, whereas others are always gases or liquids.
Changing States
Matter constantly goes through cycles that involve changing states. Water and all the elements important to organisms, including carbon and nitrogen, are constantly recycled on Earth (see Principles of Ecology). As matter moves through its cycles, it changes state repeatedly. For example, in the water cycle, water repeatedly changes from a gas to a liquid or solid and back to a gas again. How does this happen?
Adding energy to matter gives its atoms or molecules the ability to resist some of the forces holding them together. For example, heating ice to its melting point (0°C) gives its molecules enough energy to move. The ice melts and becomes liquid water. Similarly, heating liquid water to its boiling point (100°C) gives its molecules enough energy to pull apart from one another so they no longer have contact. The liquid water vaporizes and becomes water vapor.
Lesson Summary
Matter consists of elements and compounds. A compound forms when elements com- bine in fixed proportions and undergo a chemical reaction. A mixture forms when substances combine in any proportions without a chemical reaction.
Energy is a property of matter. It cannot be created or destroyed. Organisms obtain light energy from sunlight or chemical energy from food and change the energy into different forms, including heat energy.
Matter can exist in one of several different states, including a gas, liquid, or solid state. States of matter differ in the amount of energy their molecules have. When matter recycles, it changes state by gaining or losing energy.
Review Questions
Further Reading / Supplemental Links
Define element, and give an example of an element.
State how a compound differs from an element, and give an example of a compound.
What is energy?
What are three common states of matter?
Describe two ways that energy changes form in the following sequence of events.
A plant grows in the sun. → A rabbit eats the plant.
Describe a real-life situation in which the energy of an object or person changes back and forth between kinetic energy and potential energy. Identify each time energy changes form.
Compare and contrast mixtures and compounds.
Explain what happens to molecules of matter when matter changes state from a liquid to a gas.
David Bodanis, E = mc2: A Biography of the World’s Most Famous Question. Walker and Co., 2005.
John Emsley, Nature’s Building Blocks: An A-Z Guide to the Elements. Oxford Uni- versity Press, 2003.
Nevin Katz, Elements, Compounds, and Mixtures: Middle and High School (Mr. Bird- ley Teaches Science). Incentive Publications, 2007.
Vocabulary
chemical compound Unique substance with a fixed composition that forms when atoms of two or more elements react.
element Pure substance made up of just one type of atom.
energy Property of matter that is defined as the ability to do work.
gas State of matter in which atoms or molecules have enough energy to move freely.
kinetic energy Form of energy that an object has when it is moving.
liquid State of matter in which atoms or molecules are constantly in contact but have enough energy to keep changing positions relative to one another.
matter All the substances of which things are made.
mixture Combination of chemical substances that does not have a fixed composition and does not result from a chemical reaction.
organic compound Type of chemical compound that contains carbon and hydrogen and is found mainly in organisms.
potential energy Form of energy that is stored in an object due to its position.
solid State of matter in which atoms or molecules do not have enough energy to move.
state of matter Condition that matter is in, depending on how much energy its atoms or molecules have.
Points to Consider
Like all living things, you contain many organic compounds. For example, your brain is using the organic compound glucose as you read these words. Glucose provides brain cells with energy.
What are some other organic compounds in your body?
What roles do you think other organic compounds might play?
Why are organic compounds able to carry out these roles?
How do organic compounds differ from inorganic compounds?
- Log in or register to post comments
- Email this page
